

Chiral Phosphoric Acid-Catalyzed Asymmetric Cascade Reaction of C(3) Substituted Indoles and Methyl Vinyl Ketone
Received date: 2014-06-30
Online published: 2014-08-27
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2010CB833300), the National Natural Science Foundation of China (Nos. 21025209, 21121062, 21272253, 21332009) and the Chinese Academy of Sciences.
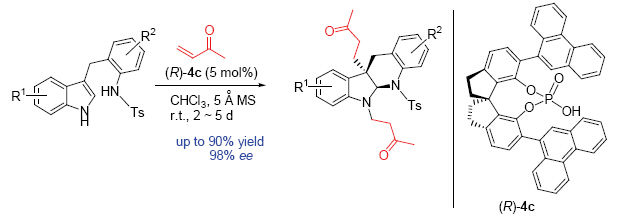
Fused indolines bearing a chiral quaternary carbon center at the C(3) position represent very important moieties widely existed in natural products and biologically active compounds. Various approaches to access these important scaffolds have been developed. Among them, asymmetric dearomatization\cyclization cascade reaction is the most concise and effective method by using indole derivatives as starting material. However, the documented reports mainly employed tryptamine or tryptophol derivatives, providing indoline products, such as pyrroloindolines or furoindolines. Therefore, the development of other type of indole derivatives allowing structural diversity is highly desirable. In this paper, an efficient asymmetric Michael addition\cyclization cascade reaction of indole derivatives with methyl vinyl ketone (MVK) was developed. After screening various phosphoric acids in the reaction of indole derivative (1a) with MVK, this cascade reaction delivered the dearomative product (2a) in 77% yield and 95% enantiomeric excess in the presence of (R)-SPINOL-derived chiral phosphoric acid (R)-4c with 5 Å molecular sieves as additive in CHCl3 at room temperature. Under the optimized reaction conditions, a wide range of substituted indole derivatives bearing both electron-donating and electron-withdrawing groups have been tested. In all cases, the cascade dearomatization reaction proceeded smoothly to afford their corresponding indolo[2,3-b]quinoline products in moderate to good yields and excellent enantioselectivity. The absolute configuration of the products was then determined as (5aR,10bR) by an X-ray crystallographic analysis of a single crystal of enantiopure 2m. Moreover, this catalytic system was also feasible in a gram-scale reaction without erosion of enantiomeric excess. 1.25 g of product 2a can be prepared under the identical conditions in 91% yield and 93% ee.
Duan Dehe , Yin Qin , Wang Shouguo , Gu Qing , You Shuli . Chiral Phosphoric Acid-Catalyzed Asymmetric Cascade Reaction of C(3) Substituted Indoles and Methyl Vinyl Ketone[J]. Acta Chimica Sinica, 2014 , 72(9) : 1001 -1004 . DOI: 10.6023/A14060497
[1] (a) Takano, S.; Ogasawara, K. Alkaloids 1989, 36, 225.
(b) Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed., Wiley, New York, 2002, pp. 1~507.
(c) Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Eds.: Fattorusso, E.; Taglialatela-Scafati, O., Wiley-VCH, Weinheim, 2008.
[2] For recent reviews, see:
(a) Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608.
(b) Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Chem. Soc. Rev. 2010, 39, 4449.
(c) Zhuo, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662. For a recent highlight, see:
(d) Loh, C. C. J.; Enders, D. Angew. Chem., Int. Ed. 2012, 51, 46.
[3] For selected recent organocatalytic asymmetric dearomatization reactions, see:
(a) Austin, J. F.; Kim, S.-G.; Sinz, C. J.; Xiao, W.-J.; MacMillan, D. W. C. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5482.
(b) Jones, S. B.; Simmons, B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 13606.
(c) Zheng, C.; Lu, Y.; Zhang, J.; Chen, X.; Chai, Z.; Ma, W.; Zhao, G. Chem. Eur. J. 2010, 16, 5853.
(d) Xiao, Y.-C.; Wang, C.; Yao, Y.; Sun, J.; Chen, Y.-C. Angew. Chem., Int. Ed. 2011, 50, 10661.
(e) Jones, S. B.; Simmons, B.; Mastracchio, A.; MacMillan, D. W. C. Nature 2011, 475, 183.
(f) Zhang, Z.; Antilla, J. C. Angew. Chem., Int. Ed. 2012, 51, 11778.
(g) Wei, Q.; Wang, Y.-Y.; Du, Y.-L.; Gong, L.-Z. Beilstein J. Org. Chem. 2013, 9, 1559.
(h) Xie, W.; Jiang, G.; Liu, H.; Hu, J.; Pan, X.; Zhang, H.; Wan, X.; Lai, Y.; Ma, D. Angew. Chem., Int. Ed. 2013, 52, 12924. For selected recent metal-catalyzed asymmetric dearomatization reactions, see:
(i) Trost, B. M.; Quancard, J. J. Am. Chem. Soc. 2006, 128, 6314.
(j) Barluenga, J.; Tudela, E.; Ballesteros, A.; Tomás, M. J. Am. Chem. Soc. 2009, 131, 2096.
(k) Benkovics, T.; Guzei, I. A.; Yoon, T. P. Angew. Chem., Int. Ed. 2010, 49, 9153. (l) Repka, L. M.; Ni, J.; Reisman, S. E. J. Am. Chem. Soc. 2010, 132, 14418. (m) Zhu, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2012, 134, 10815. (n) Spangler, J. E.; Davies, H. M. L. J. Am. Chem. Soc. 2013, 135, 6802. (o) Xiong, H.; Xu, H.; Liao, S.; Xie, Z.; Tang, Y. J. Am. Chem. Soc. 2013, 135, 7851.
[4] For selected examples from this group:
(a) Cai, Q.; Zheng, C.; Zhang, J.-W.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 8665.
(b) Cai, Q.; You, S.-L. Org. Lett. 2012, 14, 3040.
(c) Cai, Q.; Liu, C.; Liang, X.-W.; You, S.-L. Org. Lett. 2012, 14, 4588.
(d) Yin, Q.; You, S.-L. Org. Lett. 2013, 15, 4266.
(e) Yin, Q.; You, S.-L. Org. Lett. 2014, 16, 2426.
(f) Zhang, X.; Han, L.; You, S.-L. Chem. Sci. 2014, 5, 1059.
(g) Han, L.; Liu, C.; Zhang, W.; Shi, X.-X.; You, S.-L. Chem. Commun. 2014, 50, 1231.
(h) Cai, Q.; Yin, Q.; You, S.-L. Asian J. Org. Chem. 2014, 3, 408.
[5] (a) Robertson, F. J.; Kenimer, B. D.; Wu, J. Tetrahedron 2011, 67, 4327.
(b) Lin, A.; Yang, J.; Hashim, M. Org. Lett. 2013, 15, 1950.
(c) An, J.; Zou, Y.-Q.; Yang, Q.-Q.; Wang, Q.; Xiao, W.-J. Adv. Synth. Catal. 2013, 355, 1483.
[6] For early contributions, see:
(a) Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem., Int. Ed. 2004, 43, 1566.
(b) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356. For selected reviews, see:
(c) Akiyama, T. Chem. Rev. 2007, 107, 5744.
(d) You, S.-L.; Cai, Q.; Zeng, M. Chem. Soc. Rev. 2009, 38, 2190.
(e) Terada, M. Synthesis 2010, 12, 1929.
(f) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
(g) Schenker, S.; Zamfir, A.; Freund, M.; Tsogoeva, S. B. Eur. J. Org. Chem. 2011, 2209.
(h) Wu, X.; Li, M.; Gong, L. Acta Chim. Sinica 2013, 71, 1091. (吴祥, 李明丽, 龚流柱, 化学学报, 2013, 71, 1091.)
[7] For selected examples on spinol-derived chiral phosphoric acids:
(a) ?ori?, I.; Müller, S.; List, B. J. Am. Chem. Soc. 2010, 132, 17370.
(b) Xu, F.; Huang, D.; Han, C.; Shen, W.; Lin, X.; Wang, Y. J. Org. Chem. 2010, 75, 8677.
(c) Müller, S.; Webber, M. J.; List, B. J. Am. Chem. Soc. 2011, 133, 18534.
(d) Xing, C.-H.; Liao, Y.-X.; Ng, J.; Hu, Q.-S. J. Org. Chem. 2011, 76, 4125.
(e) Xu, B.; Zhu, S.-F.; Xie, X.-L.; Shen, J.-J.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2011, 50, 11483.
(f) Wang, S.-G.; You, S.-L. Angew. Chem., Int. Ed. 2014, 53, 2194.
/
| 〈 |
|
〉 |