

Copper-Catalyzed Benzylic sp3 C-H Amination Reaction of Amidines: Synthesis of Quinazoline Derivatives
Received date: 2014-08-14
Online published: 2014-10-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172033, 21372041) and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110043110002).
Quinazoline motif is an important heterocyclic framework, which widely exists in many biologically active molecules and natural products. Although numerous synthetic efforts have been made for the preparation of quinazoline derivatives in recent years, it is still highly desirable to search for a more convenient and efficient approach. As part of our ongoing interest in transition-metal-catalyzed C—H functionalization, herein, we report a novel copper-catalyzed synthesis of quinazoline derivatives from amidines. During these transformations, the selectivity of benzylic sp3 C—H over aryl sp2 C—H bond was efficiently realized. Various 2-arylquinazoline derivatives were obtained in moderate to good yields. A representative procedure for the copper-catalyzed construction of quinazolines from amidines is as following: N-(o-tolyl)benzimidamide (1a, 0.4 mmol, 89.7 mg), Cu(OTf)2 (0.04 mmol, 13.8 mg) and Ag2CO3 (0.8 mmol, 219 mg) were added to a 25 mL sealed tube, followed by addition of DMF (2 mL). The mixture was stirred at 140 ℃ for 10 h. After cooling to room temperature, the mixture was poured into ice-water and extracted with CH2Cl2 (15 mL×3). The combined organic layers were dried (Na2SO4), filtered over Celite, evaporated in vacuo. The residue was purified by a shot flash silica gel column chromatography (petroleum ether/ethyl acetate, V:V=15:1) to afford the product 2a (71%, 62.4 mg).
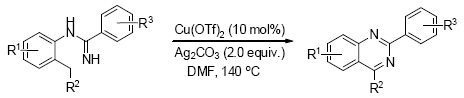
Key words: copper; N-arylamidines; C—N bond formation; quinazoline derivatives
Zhang Qian, Lü Yunhe, Li Yan, Xiong Tao, Zhang Qian . Copper-Catalyzed Benzylic sp3 C-H Amination Reaction of Amidines: Synthesis of Quinazoline Derivatives[J]. Acta Chimica Sinica, 2014 , 72(11) : 1139 -1143 . DOI: 10.6023/A14080584
[1] (a) Henderson, E. A.; Bavetsias, V.; Theti, D. S.; Wilson, S. C.; Clauss, R.; Jackman, A. L. Bioorg. Med. Chem. 2006, 14, 5020.
(b) Doyle, L. A.; Ross, D. D. Oncogene 2003, 22, 7340.
(c) Foster, A.; Coffrey, H. A.; Morin, M. J.; Rastinejad, F. Science 1999, 286, 2507.
[2] (a) Chien, T. C.; Chen, C. S.; Yu, F. H.; Chern, J. W. Chem. Pharm. Bull. 2004, 52, 1422.
(b) Herget, T.; Freitag, M.; Morbitzer, M.; Kupfer, R.; Stamminger, T.; Marschall, M. Antimicrob. Agents Chemother. 2004, 48, 4154.
[3] (a) Waisser, K.; Gregor, J.; Dostal, H.; Kunes, J.; Kubicova, L.; Klimesova, V.; Kaustova, J. Farmaco 2001, 56, 803.
(b) Kunes, J.; Bazant, J.; Pour, M.; Waisser, K.; Slosarek, M.; Janota, J. Farmaco 2000, 55, 725.
[4] For recent reviews about the synthesis of quinazolines and its derivatives, see: (a) Connolly, D. J.; Cusack, D.; O’Sullivan, T. P.; Guiry, P. J. Tetrahedron 2005, 61, 10153.
(b) Michael, J. P. Nat. Prod. Rep. 2007, 24, 223. For the recent reports of quinazolines, see:
(c) Deng, L. Q.; Zhong, H.; Wang, S. Chin. J. Org. Chem. 2014, 34, 414. (邓兰青, 钟宏, 王帅, 有机化学, 2014, 34, 414.)
(d) Yang, X. H.; Wang, X.; Wu, M. H. Chin. J. Org. Chem. 2014, 34, 1015. (杨绪红, 王翔, 吴鸣虎, 有机化学, 2014, 34, 1015.)
(e) Liu, H. B.; Xu, H. J.; Lü, P.; Pan, N. N.; Li, S. Q. Acta Chim. Sinica 2012, 70, 674. (刘海彬, 徐惠娟, 吕萍, 潘宁宁, 李双奇, 化学学报, 2012, 70, 674.)
(f) Zhang, M. M.; Liu, Y.; Wang, X. S. Chin. J. Org. Chem. 2014, 34, 1682. (张梅梅, 刘蕴, 王香善, 有机化学, 2014, 34, 1682.)
(g) Ou, J. J.; Liu, K. C.; Wang, Y.; Zhang, H.; Liu, R. Q.; Li, Q. B.; Wang, Q. M.; Li, Y. Q.; Rui, C. H.; Liu, S. Z. Chin. J. Org. Chem. 2014, 34, 526 (欧俊军, 刘克昌, 王毅, 张浩, 刘瑞全, 李奇博, 汪清民, 李永强, 芮昌辉, 刘尚钟, 有机化学, 2014, 34, 526.)
(h) Liu, J. H.; Liu, Y.; Jian, J. Y.; Bao, X. P. Chin. J. Org. Chem. 2013, 33, 370. (刘军虎, 刘勇, 蹇军友, 鲍小平, 有机化学, 2013, 33, 370.)
[5] (a) Shi, W.; Liu, C.; Lei, A. W. Chem. Soc. Rev. 2011, 40, 2761.
(b) Liu, C.; Zhang, H.; Shi, W.; Lei, A. W. Chem. Rev. 2011, 111, 1780.
(c) Zhao, Y. S.; Wang, H. B.; Hou, X. H.; Hu, Y. H.; Lei, A. W.; Zhang, H.; Zhu, L. Z. J. Am. Chem. Soc. 2006, 128, 15049.
(d) Liu, C.; Jin, L. Q.; Lei, A. W. Synlett 2010, 17, 2527.
[6] (a) Tang, C. H.; Yuan, Y. Z.; Cui, Y. X.; Jiao, N. Eur. J. Org. Chem. 2013, 7480.
(b) Zhang, C.; Xu, Z. J.; Zhang, L. R.; Jiao, N. Angew. Chem., Int. Ed. 2011, 50, 11088.
(c) Zhang, C.; Xu, Z. J.; Zhang, L. R.; Jiao, N. Tetrahedron 2012, 68, 5258.
(d) Xu, Z. J.; Zhang, C.; Jiao, N. Angew. Chem., Int. Ed. 2012, 51, 11367.
(e) Zhang, B.; Feng, P.; Sun, L. H.; Cui, Y. X.; Ye, S.; Jiao, N. Chem. Eur. J. 2012, 18, 9198.
(f) Wu, G.; Su, W. P. Org. Lett. 2013, 15, 5278.
(g) Zhao, H. Q.; Shang, Y. P.; Su, W. P. Org. Lett. 2013, 15, 5106.
(h) Lü, T. Y.; Wang, Z.; You, J. S.; Lan, J. B.; Gao, G. J. Org. Chem. 2013, 78, 5723.
(i) Li, X. Y.; Li, B. J.; You, J. S.; Lan, J. B. Org. Biomol. Chem. 2013, 11, 1925.
[7] (a) Ma, Y. Y.; Li, W.; Yu, B. Acta Chim. Sinica 2013, 71, 541. (马玉勇, 李微, 俞飚, 化学学报, 2013, 71, 541.)
(b) Pan, F.; Shi, Z. J. Acta Chim. Sinica 2012, 70, 1679. (潘菲, 施章杰, 化学学报, 2012, 70, 1679.)
(c) Liu, D. J.; Yu, H. Z.; Fu, Y. Acta Chim. Sinica 2013, 71, 1385. (刘丁嘉, 于海珠, 傅尧, 化学学报, 2013, 71, 1385.)
[8] (a) Nguyen, Q.; Sun, K.; Driver, T. G. J. Am. Chem. Soc. 2012, 134, 7262.
(b) Neumann, J. J.; Rakshit, S.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2009, 48, 6892.
(c) Shi, R. Y.; Lu, L. J.; Zhang, H.; Chen, B. R.; Sha, Y. C.; Liu, C.; Lei, A. W. Angew. Chem., Int. Ed. 2013, 52, 10582.
[9] (a) Nadres, E. T.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 7.
(b) He, G.; Zhao, Y.; Zhang, S.; Lu, C.; Chen, G. J. Am. Chem. Soc. 2012, 134, 3.
(c) Sun, K.; Sachwani, R.; Richert, K. J.; Driver, T. G. Org. Lett. 2009, 11, 3598.
(d) Yang, L.; Ma, Y. H.; Song, F. J.; You, J. S. Chem. Commun. 2014, 50, 3024.
[10] (a) Ichinose, M.; Suematsu, H.; Yasutomi, Y.; Nishioka, Y.; Uchida, T.; Katsuki, T. Angew. Chem., Int. Ed. 2011, 50, 9884.
(b) Ruppel, J. V.; Kamble, R. M.; Zhang, X. P. Org. Lett. 2007, 9, 4889.
[11] Wang, Y. F.; Chen, H.; Zhu, X.; Chiba, S. J. Am. Chem. Soc. 2012, 134, 11980.
[12] (a) Wang, Z.; Ni, J.; Kuninobu, Y.; Kanai, M. Angew. Chem., Int. Ed. 2014, 53, 3496,
(b) Li, W.; Liu, C.; Zhang, H.; Ye, K. Y.; Zhang, G. H.; Zhang, W. Z.; Duan, Z. L.; You, S. L.; Lei, A. W. Angew. Chem., Int. Ed. 2014, 53, 2443
[13] (a) Brasche, G.; Buchwald, S. L. Angew. Chem., Int. Ed. 2008, 47, 1932.
(b) Xiao, Q.; Wang, G.; Liu, W. H.; Meng, F. K.; Chen, J. H.; Yang, Z.; Shi, Z. J. Chem. Eur. J. 2009, 15, 7292.
(c) Wang, H. G.; Wang, Y.; Peng, C. L.; Zhang, J. C.; Zhu, Q. J. Am. Chem. Soc. 2010, 132, 13217.
[14] (a) Gao, M.; He, C.; Chen, H. Y.; Bai, R. P.; Cheng, B.; Lei, A. W. Angew. Chem., Int. Ed. 2013, 52, 6958.
(b) Meng, L. K.; Wu, K.; Liu, C.; Lei, A. W. Chem. Commun. 2013, 49, 5853.
(c) Ke, J.; He, C.; Liu, H. Y.; Li, M. J.; Lei, A. W. Chem. Commun. 2013, 49, 7549.
[15] (a) Li, L. J.; Zhang, Y. Q.; Zhang, Y.; Zhu, A. L.; Zhang, G. S. Chin. Chem. Lett. 2014, 25, 1161.
(b) Wang, H. B.; Wang, L.; Shang, J. S.; Li, X.; Wang, H. Y.; Gui, J.; Lei, A. W. Chem. Commun. 2012, 48, 76.
(c) Liu, J.; Fan, C.; Yin, H. Y.; Qin, C.; Zhang, G. T.; Zhang, X.; Yi, H.; Lei, A. W. Chem. Commun. 2014, 50, 2145.
(d) He, C.; Hao, J.; Xu, H.; Mo, Y. P.; Liu, H. Y.; Hao, J. J.; Lei, A. W. Chem. Commun. 2012, 48, 11073.
(e) Gao, M.; Tian, J.; Lei, A. W. Chem. Asian J. 2014, 9, 2068.
[16] (a) Zhang, X. Y.; Fang, L. L.; Liu, N.; Wu, H. Y.; Fang, X. S. Chin. Chem. Lett. 2012, 23, 1129.
(b) He, C.; Guo, S.; Ke, J.; Hao, J.; Xu, H.; Chen, H. Y.; Lei, A. W. J. Am. Chem. Soc. 2012, 134, 5766.
(c) Huang, Z. L.; Jin, L. Q.; Feng, Y.; Peng, P.; Yi, H.; Lei, A. W. Angew. Chem., Int. Ed. 2013, 52, 7151.
(d) Meng, L. K.; Zhang, G. H.; Liu, C.; Wu, K.; Lei, A. W. Angew. Chem., Int. Ed. 2013, 52, 10195
[17] (a) Xiong, T.; Li, Y.; Bi, X.; Lv, Y. H.; Zhang, Q. Angew. Chem., Int. Ed. 2011, 50, 7140.
(b) Li, Y.; Li, Z. S.; Xiong, T.; Zhang, Q.; Zhang, X. Y. Org. Lett. 2012, 14, 3522.
[18] (a) Sun, K.; Li, Y.; Xiong, T.; Zhang, J. P.; Zhang, Q. J. Am. Chem. Soc. 2011, 133, 1694.
(b) Xiong, T.; Li, Y.; Lv, Y. H.; Zhang, Q. Chem. Commun. 2010, 46, 6831.
(c) Ni, Z. K.; Zhang, Q.; Xiong, T.; Zheng, Y. Y.; Li, Y.; Zhang, H. W.; Zhang, J. P.; Liu, Q. Angew. Chem., Int. Ed. 2012, 51, 1244.
(d) Xiong, T.; Li, Y.; Mao, L. J.; Zhang, Q.; Zhang, Q. Chem. Commun. 2012, 48, 2246.
(e) Zhang, H. W.; Pu, W. Y.; Xiong, T.; Li, Y.; Zhou, X.; Sun, K.; Liu, Q. Zhang, Q. Angew. Chem., Int. Ed. 2013, 52, 2529.
(f) Lv, Y. H.; Li, Y.; Xiong, T.; Pu, W. Y.; Zhang, H. W.; Sun, K.; Liu, Q.; Zhang, Q. Chem. Commun. 2013, 49, 6439.
(e) Lv, Y. H.; Zheng, Y. Y.; Li, Y.; Xiong, T.; Zhang, J. P.; Liu, Q.; Zhang, Q. Chem. Commun. 2013, 49, 8866.
[19] Bhadra, S.; Matheis, C.; Katayev, D.; Gooßen, L. J. Angew. Chem., Int. Ed. 2013, 52, 9279.
[20] Singh, B.; Collins, J. C. Chem. Commun. 1971, 498.
/
| 〈 |
|
〉 |