

Use of Ionic Liquid 1-Octyl-3-methylimidazolium Hexafluorophosphate as a Recyclable Solvent for Extraction/Removal of Hg(Ⅱ) from Water
Received date: 2014-08-15
Online published: 2014-09-30
Supported by
Project supported by the National Natural Science Foundation of China (No. 31301474) and the Public Service Applications of Beijing Centers for Preventive Medical Research (No. 2014-BJYJ-12).
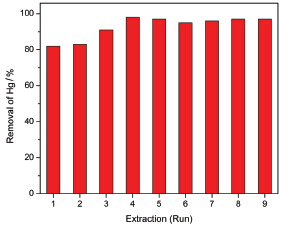
Mercury, a highly persistent and toxic substance, seriously threatens the human health and the environment. As an environmental friend solvent, ionic liquids (ILs) have been used as replacements for conventional organic solvents in various separation processes. But there are still some technical aspects that need to be addressed before ILs can be practically applied to remove Hg(Ⅱ) from water. The first and most important issue is the recovery and recycling of ILs without significant loss to make the process economically viable and reduce their potential environmental burden. In this study, a novel approach for removal of Hg(Ⅱ) from water was developed via 1-octyl-3-methylimidazolium hexafluorophosphate ([C8MIM]PF6). Hg(Ⅱ) was extracted into[C8MIM]PF6 and then reduced into Hg(0) with formic acid. Extraction and reduction conditions were optimized. Because the extraction efficiency of Hg(Ⅱ) into the pristine[C8MIM]PF6 was limited, 0.4% (V/V) 1-methylimidazole was added as complex agent of Hg(Ⅱ) into[C8MIM]PF6 to enhance the extraction efficiency. Under a phase ratio of[C8MIM]PF6 to water of 1:50, almost 100% of the Hg(Ⅱ) in water phase (1 mg/L) was extracted into the[C8MIM]PF6 phase after shaking for 2 h at 220 r/min and 50 ℃. Afterwards, Hg(Ⅱ) concentrated in the IL was reduced to Hg(0) by formic acid under the heating condition, and then the[C8MIM]PF6 was isolated for the purpose of recycling the IL. The Hg(Ⅱ) in[C8MIM]PF6 was reduced to Hg(0) using 40% (V/V) formic acid at 50 ℃. Under a phase ratio of 40% (V/V) formic acid to[C8MIM]PF6 of 4:1, about 60%~70% of Hg(Ⅱ) was reduced to Hg(0) in 20 min. The formed Hg(0) in the mixture was removed after centrifugation, and the[C8MIM]PF6 was collected and recycled. The extraction efficiency for Hg(Ⅱ) in water was ca. 83% in the initial two runs, and then increased to 91%~98% in the following 7 runs by the attribution of the formic acid which is residues in the IL phase and enhances extraction efficiency.
Key words: ionic liquid; mercury; liquid-liquid extraction; reduction; formic acid
Wang Xiaowei , Chen Sha . Use of Ionic Liquid 1-Octyl-3-methylimidazolium Hexafluorophosphate as a Recyclable Solvent for Extraction/Removal of Hg(Ⅱ) from Water[J]. Acta Chimica Sinica, 2014 , 72(11) : 1147 -1151 . DOI: 10.6023/A14080587
[1] Nolan, E. M.; Lippard, S. J. Chem. Rev. 2008, 108, 3443.
[2] Tchounwou, P. B.; Ayensu, W. K.; Ninashvili, N.; Sutton, D. Environ. Toxicol. 2003, 18, 149.
[3] Nriagu, J. O. Nature 1989, 338, 47.
[4] Onyido, I.; Norris, A. R.; Buncel, E. Chem. Rev. 2004, 104, 5911.
[5] Mureseanu, M.; Reiss, A.; Cioatera, N.; Trandafir, I.; Hulea, V. J. Hazard. Mater. 2010, 182, 197.
[6] Goyal, M.; Bhagat, M.; Dhawan, R. J. Hazard. Mater. 2009, 171, 1009.
[7] Zabihi, M.; Ahmadpour, A.; Asl, A. H. J. Hazard. Mater. 2009, 167, 230.
[8] Liu, J. F.; Zhao, Z. S.; Jiang, G. B. Environ. Sci. Technol. 2008, 42, 6949.
[9] Papaiconomou, N.; Lee, J. M.; Salminen, J.; Von Stosch, M.; Prausnitz, J. M. Ind. Eng. Chem. Res. 2007, 47, 5080.
[10] Germani, R.; Mancini, M. V.; Savelli, G.; Spreti, N. Tetrahedron Lett. 2007, 48, 1767.
[11] Visser, A. E.; Swatloski, R. P.; Reichert, W. M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J. H.; Rogers, R. D. Environ. Sci. Technol. 2002, 36, 2523.
[12] Ersoz, M. Adv. Colloid Interface Sci. 2007, 134, 96.
[13] Gao, Z.; Ma, X. Anal. Chim. Acta 2001, 702, 50.
[14] Holbrey, J. D.; Visser, A. E.; Spear, S. K.; Reichert, W. M.; Swatloski, R. P.; Brokera, G. A.; Rogersa, R. D. Green Chem. 2003, 5, 129.
[15] Jin, Z.; Xie, D. X.; Zhang, X. B.; Gong, Y. J.; Tan, W. Anal. Chem. 2012, 84, 4253.
[16] Gkika, E.; Troupis, A.; Hiskia, A.; Papaconstantinou, E. Environ. Sci. Technol. 2005, 39, 4242.
[17] Ribeiro, A. S.; Vieira, M. A.; Willie, S.; Sturgeon, R. E. Anal. Bioanal. Chem. 2007, 388, 849.
[18] Gil, S.; Lavilla, I.; Bendicho, C. Ultrason. Sonochem. 2008, 15, 212.
[19] Liang, L. N.; Jiang, G. B.; Liu, J. F.; Hu, J. T. Anal. Chim. Acta 2003, 477, 131.
[20] Visser, A. E.; Swatloski, R. P.; Griffin, S. T.; Hartman, D. H.; Rogers, R. D. Sep. Sci. Technol. 2001, 36, 785.
[21] Chen, S.; Sun, H.; Zhong, Y.-S. J. Beijing University of Technology 2013, 39, 98. (陈莎, 孙浩, 钟嶷盛, 北京工业大学学报, 2013, 39, 98.)
[22] Rivas, B. L.; Jara, M.; Pereira, E. D. J. Appl. Polym. Sci. 2003, 89, 2852.
/
| 〈 |
|
〉 |