

Doxorubicin-Loaded Silk Fibroin Nanospheres
Received date: 2014-08-21
Online published: 2014-10-13
Supported by
Project supported by the Specialized Research Fund for the Doctoral Program of Higher Education, MOE of China (No. 20110071110008) and the National Natural Science Foundation of China (No. 21274028).
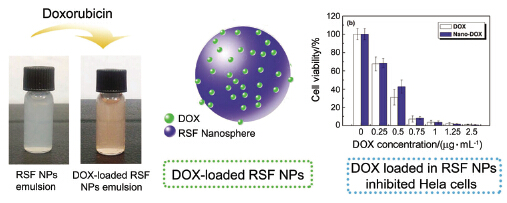
Silk protein from silkworms or spiders is a very promising biomaterial due to its renewability, nontoxicity, biocompatibility and biodegradability, so it has been widely used in biomedical and pharmaceutical fields. In this article, we report our attempt to use regenerated Bombyx mori silkworm silk fibroin (RSF) as a drug-carrier to encapsulate anti-cancer drug doxorubicin (DOX). Firstly, the pristine RSF nanospheres are prepared by using a facile and clean method developed in this laboratory previously based on the self-assembly of silk protein. In brief, after adding a small amount of ethanol into RSF solution, freezing the whole system to -20 ℃ for 24 h, and then defreezing at room temperature. These RSF nanospheres almost have no cytotoxicity because there is no additional organic solvent other than ethanol involved in the preparation process. Afterwards, the DOX-loaded RSF nanospheres with the average sizes ranging from 350 to 400 nm are prepared by simply mixing DOX aqueous solution and RSF nanospheres solution. The characterizations from dynamic light scattering and SEM observation show that DOX-loaded RSF nanospheres have a controllable shape and size, without apparent aggregation. The drug loading is about 4.6%, the encapsulation efficiency is more than 90%, and the release time of such kind of DOX-loaded RSF nanospheres is over 7 days. In addition, these DOX loaded SF-nanospheres show pH-dependent release, that is, the drug releases faster in pH=5.0 buffer solution than that in pH=7.4 one. The DOX-loaded in the RSF nanospheres exhibits the similar curative effect to kill or inhibit Hela cells to the free DOX after incubating these drug-loaded nanospheres with Hela cells for 24 or 48 h. All these results, including easy preparation, good biocompatibility, suitable particle size, and considerable anti-cancer efficiency, imply that such kind of biomacromolecule based anti-cancer drug nanocarrier has a great potential for the lymphatic chemotherapy in clinical applications.
Yang Wenhua , Yu Shuying , Chen Sheng , Liu Yezhuo , Shao Zhengzhong , Chen Xin . Doxorubicin-Loaded Silk Fibroin Nanospheres[J]. Acta Chimica Sinica, 2014 , 72(11) : 1164 -1168 . DOI: 10.6023/A14080596
[1] Williams, D. F. Biomaterials 2008, 29, 2941.
[2] Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. J. Controlled Release 2013, 166, 182.
[3] Elzoghby, A. O.; Samy, W. M.; Elgindy, N. A. J. Controlled Release 2012, 161, 38.
[4] Wang, Y. Z.; Kim, H. J.; Vunjak-Novakovic, G.; Kaplan, D. L. Biomaterials 2006, 27, 6064.
[5] Jiang, X. R.; Guan, J.; Chen, X.; Shao, Z. Z. Acta Chim. Sinica 2010, 68, 1909. (江霞蓉, 管娟, 陈新, 邵正中, 化学学报, 2010, 68, 1909.)
[6] Ma, X. Y.; Shi, L. J.; Zhou, J.; Zhu, J.; Zhong, J.; Wei, R. L. Chin. J. Org. Chem. 2013, 33, 1080. (马晓晔, 施丽君, 周涓, 朱君, 钟建, 魏锐利, 有机化学, 2013, 33, 1080.)
[7] Wei, Q. N.; Ma, L.; Huang, A. M.; Yang, H.; Gong, Z. P.; Qiang, P. P.; Zhang, L. Acta Chim. Sinica 2012, 70, 714. (韦俏娜, 马林, 黄爱民, 杨华, 龚珠萍, 强盼盼, 张丽, 化学学报, 2012, 70, 714.)
[8] Cao, Z. B.; Chen, X.; Yao, J. R.; Huang, L.; Shao, Z. Z. Soft Matter 2007, 3, 910.
[9] Moghimi, S. M.; Hunter, A. C.; Murray, J. C. Pharm. Rev. 2001, 53, 283.
[10] Swartz, M. A. Adv. Drug Delivery Rev. 2001, 50, 3.
[11] Oussoren, C.; Storm, G. Adv. Drug Delivery Rev. 2001, 50, 143.
[12] Liu, J.; Wong, H. L.; Moselhy, J.; Bowen, B.; Wu, X. Y.; Johnston, M. R. Lung Cancer 2006, 51, 377.
[13] Nishioka, Y.; Yoshino, H. Adv. Drug Delivery Rev. 2001, 47, 55.
[14] Liu, J.; Meisner, D.; Kwong, E.; Wu, X. Y.; Johnston, M. R. Biomaterials 2007, 28, 3236.
[15] Lee, S.; Baek, M.; Kim, H.-Y.; Ha, J.-H.; Jeoung, D.-I. Biotechnol. Lett. 2002, 24, 1147.
[16] He, H.; Wang, Y.; Wen, H.; Jia, X. RSC Adv. 2014, 4, 3643.
[17] Carvalho, F. S.; Burgeiro, A.; Garcia, R.; Moreno, A. J.; Carvalho, R. A.; Oliveira, P. J. Med. Res. Rev. 2014, 34, 106.
[18] Basuki, J. S.; Duong, H. T.; Macmillan, A.; Erlich, R. B.; Esser, L.; Akerfeldt, M. C.; Whan, R. M.; Kavallaris, M.; Boyer, C.; Davis, T. P. ACS Nano 2013, 7, 10175.
[19] Guo, Q. Q.; Wang, H. Y.; Zhao, Y. X.; Wang, H. X.; Zeng, F.; Hua, H. Y.; Xu, Q.; Huang, Y. Z. Polym. Chem. 2013, 4, 4584.
[20] Sanyakamdhorn, S.; Agudelo, D.; Tajmir-Riahi, H.-A. Biomacromolecules 2013, 14, 557.
[21] Liu, Z.; Sun, X. M.; Nakayama-Ratchford, N.; Dai, H. J. ACS Nano 2007, 1, 50.
[22] Yu, S. Y.; Yang, W. H.; Chen, S.; Chen, M. J.; Liu, Y. Z.; Shao, Z. Z.; Chen, X. RSC Adv. 2014, 4, 18171.
[23] Chen, M. J.; Shao, Z. Z.; Chen, X. J. Biomed. Mater. Res. A 2012, 100, 203.
[24] Chan, J. M.; Zhang, L. F.; Yuet, K. P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O. C. Biomaterials 2009, 30, 1627.
[25] Cheng, J. J.; Teply, B. A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F. X.; Levy-Nissenbaum, E.; Radovic-Moreno, A. F.; Langer, R.; Farokhzad, O. C. Biomaterials 2007, 28, 869.
[26] Allen, C.; Dos Santos, N.; Gallagher, R.; Chiu, G.; Shu, Y.; Li, W.; Johnstone, S.; Janoff, A.; Mayer, L.; Webb, M. Biosci. Rep. 2002, 22, 225.
[27] Greenwald, R. B.; Choe, Y. H.; McGuire, J.; Conover, C. D. Adv. Drug Delivery Rev. 2003, 55, 217.
[28] Ma, G. L.; Zhao, S. X.; Jin, X.; Chen, M. M.; Zhang, Z. P.; Song, C. X. Chem. J. Chin. Univ. 2012, 33, 1854 (马桂蕾, 赵顺新, 金旭, 陈旼旼, 张政朴, 宋存先, 高等学校化学学报, 2012, 33, 1854.)
[29] Waku, T.; Matsusaki, M.; Kaneko, T.; Akashi, M. Macromolecules 2007, 40, 6385.
[30] Watson, P.; Jones, A. T.; Stephens, D. J. Adv. Drug Delivery Rev. 2005, 57, 43.
/
| 〈 |
|
〉 |