

Cholic Acid/Graphene Oxide Chiral Hybrid Material: Preparation and Characterizations
Received date: 2014-08-28
Online published: 2014-10-17
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21274008, 21174010), the Funds for Creative Research Groups of China (No. 51221002) and the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP No. 20120010130002).
The importance of chiral science and chiral materials has been increasingly highlighted. Meanwhile, graphene oxide (GO) and the derivatives are emerging materials with remarkable electronic, mechanical, and thermal properties. The present study intends to chirally functionalize oxide graphene (GO) by using a biomass cholic acid (CA), by which to establish a novel type of chiral hybrid materials. Based on expandable graphite powder, slightly modified Hummers method was adopted to prepare GO, on which 3-azidopropyltrimethoxysilane (APTMS) as N3 moieties were covalently attached by taking advantage of the reaction between the alkoxy groups of APTMS and the hydroxyl and epoxide groups of GO sheets, providing GO/APTMS. Alkynylated CA was prepared by a reaction of CA and propargylamine in the presence of isobutyl chloroformate and 4-methylmorpholine taken, according to our earlier method. Then, the solution was dried over anhydrous MgSO4, filtered, concentrated, and then purified by recrystallization twice from THF/hexane. Furthermore, the product was structurally identified by FT-IR and 1H NMR. Subsequently, alkynylated CA was grafted onto the surface of GO sheets through a "click" reaction between the azido groups on GO and the ethynyl groups in the alkynylated CA to produce chirally functionalized GO. The reaction was accomplished under a nitrogen atmosphere with addition of dry DMF, GO/APTMS, Alkynylated CA, CuBr, and PMDETA at room temperature for 48 h with magnetic stirring. Then the product was obtained by centrifugation at 18000 r/min for 30 min and further cleaned for three cycles by washing with DMF under sonication and centrifugation. Finally, the resulting chiral GO hybrid was dried under vacuum overnight and subjected to characterizations including FT-IR, XRD, XPS, Raman, TG, AFM, HRTEM, CD and UV. The characterizations in combination identified the success in preparing the target chiral hybrid material.
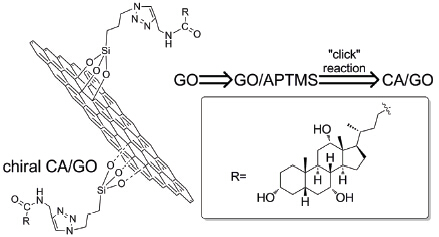
Key words: chiral materials; graphene oxide; cholic acid; click reaction
Huang Huajun , Ren Chonglei , Chen Yu , Yang Wantai , Deng Jianping . Cholic Acid/Graphene Oxide Chiral Hybrid Material: Preparation and Characterizations[J]. Acta Chimica Sinica, 2014 , 72(11) : 1169 -1174 . DOI: 10.6023/A14080614
[1] Chen, D.; Feng, H. B.; Li, J. H. Chem. Rev. 2012, 112, 6027.
[2] Konkena, B.; Vasudevan, S. J. Phys. Chem. Lett. 2012, 3, 867.
[3] Tang, Q.; Zhou, Z.; Chen, Z. Nanoscale 2013, 5, 4541.
[4] Wei, W.; Qu, K.; Ren, J.; Qu, X. Chem. Sci. 2011, 2, 2050.
[5] Loh, K. P.; Bao, Q.; Ang, P. K.; Yang, J. J. Mater. Chem. 2010, 20, 2277.
[6] Zhang, Q.; Wu, S.; Zhang, L.; Lu, J.; Verproot, F.; Liu, Y.; Xing, Z.; Li, J.; Song, X. M. Biosens. Bioelectron. 2011, 26, 2632.
[7] Fang, M.; Wang, K. G.; Lu, H. B.; Yang, Y. L.; Nutt, S. J. Mater. Chem. 2009, 19, 7098.
[8] Wang, Q.; Chan, T. R.; Hilgraf, R.; Fokin, V. V.; Sharpless, K. B.; Finn, M. J. Am. Chem. Soc. 2003, 125, 3192.
[9] Mortisen, D.; Peroglio, M.; Alini, M.; Eglin, D. Biomacromolecules 2010, 11, 1261.
[10] Yang, X.; Ma, L.; Wang, S.; Li, Y.; Tu, Y.; Zhu, X. Polymer 2011, 52, 3046.
[11] (a) Ren, C.; Chen, Y.; Zhang, H.; Deng, J. Macromol. Rapid Commun. 2013, 34, 1368.
(b) Li, W.; Liang, J.; Yang, W.; Deng, J. ACS Appl. Mater. Interfaces 2014, 6, 9790.
(c) Li, W.; Liu, X.; Qian, G.; Deng, J. Chem. Mater. 2014, 26, 1948.
[12] (a) Guo, C. X.; Zhang, L. Y.; Miao, J.; Zhang, J.; Li, C. M. Adv. Energy. Mater. 2013, 3, 167.
(b) Nasseri, M. A.; Allahresani, A.; Raissi, H. RSC Adv. 2014, 4, 26087.
(c) Hauser, A. W.; Mardirossian, N.; Panetier, J. A.; Head-Gordon, M.; Bell, A. T.; Schwerdtfeger, P. Angew. Chem., Int. Ed. 2014, 53, 1.
(d) Gu, H.; Zhou, N.-L.; Fan, Y.-T.; Wang, X.-D.; Li, W.-X.; Shen, J. Chem. J. Chin. Univ. 2013, 34, 479. (顾皓, 周宁琳, 樊云婷, 王晓丹, 李文秀, 沈健, 高等学校化学学报, 2013, 34, 479.)
(e) Mo, Z.; Gou, H.; He, J.; Yang, P.; Feng, C.; Guo, R. Appl. Surf. Sci. 2012, 258, 8623.
[13] Hofmann, A. F. News Physiol. Sci. 1999, 14, 24.
[14] Mukhopadhyay, S.; Maitra, U. Curr. Sci. India. 2004, 87, 1666.
[15] Zhu, X. X.; Nichifor, M. Acc. Chem. Res. 2002, 35, 539.
[16] Davis, A. P. Molecules 2007, 12, 2106.
[17] Meyer, J. C.; Geim, A. K.; Katsnelson, M.; Novoselov, K.; Booth, T.; Roth, S. Nature 2007, 446, 60.
[18] Oh, J.; Lee, J. H.; Koo, J. C.; Choi, H. R.; Lee, Y.; Kim, T.; Luong, N. D.; Nam, J. D. J. Mater. Chem. 2010, 20, 9200.
[19] Wang, H.; Hu, Y. H. Ind. Eng. Chem. Res. 2011, 50, 6132.
[20] Stankovich, S.; Piner, R. D.; Chen, X.; Wu, N.; Nguyen, S. T.; Ruoff, R. S. J. Mater. Chem. 2006, 16, 155.
[21] Hummers, W. S.; Offeman, R. E. J. Am. Chem. Soc. 1958, 80, 1139.
[22] Zhang, D.; Song, C.; Deng, J.; Yang, W. Macromolecules 2012, 45, 7329.
[23] Meng, D.; Sun, J.; Jiang, S.; Zeng, Y.; Li, Y.; Yan, S.; Geng, J.; Huang, Y. J. Mater. Chem. 2012, 22, 21583.
[24] Pan, Y.; Bao, H.; Sahoo, N. G.; Wu, T.; Li, L. Adv. Funct. Mater. 2011, 21, 2754.
[25] Lin, Y.-W.; Guo, X.-F. Acta Chim. Sinica 2014, 72, 277. (林源为, 郭雪峰, 化学学报, 2014, 72, 277.)
[26] Lai, C.-W.; Sun, Y.; Yang, H.; Zhang, X.-Q.; Lin, B.-P. Acta Chim. Sinica 2013, 71, 1201. (来常伟, 孙莹, 杨洪, 张雪勤, 林保平, 化学学报, 2013, 71, 1201.)
[27] Wu, J.-X.; Xu, H.; Zhang, J. Acta Chim. Sinica 2014, 72, 301. (吴娟霞, 徐华, 张锦, 化学学报, 2014, 72, 301.)
/
| 〈 |
|
〉 |