

Extraction of Copper from Aqueous Solution with Functional Ionic Liquids: Experiment and Theoretical Calculation
Received date: 2014-11-12
Online published: 2015-01-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21103047, 21136004) and the Fundamental Research Funds for the Central Universities of China (No. 222201313001).
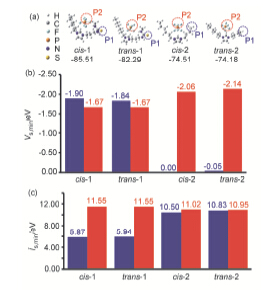
This work studied the extraction of copper (II) ions from aqueous solution with thiourea-appended imidazolium hydrophobic ionic liquids and the extraction mechanism by experiment and theory. The influence of parameters affecting the extraction of copper ion, such as the metal ion concentrations, volume ratio between aqueous solution and ionic liquid, contact time, sodium chloride and pH, as well as alkyl chain length was analyzed. In the case of room temperature, the volume ratio between aqueous/IL phases was 50, the extraction efficiencies >95% could be obtained for copper ion with all the ionic liquids [CnMPSM][PF6] (n=4, 6, 8). The results also suggest that n=4 did the same work on the extraction efficiency with n=6 but higher than n=8, while pH and the salt in the solution had little effect on the extraction efficiency for the extraction with [HMPSM][PF6]. The grafted functional group significantly enhanced the extraction efficiency for Cu2+ from 20% to over 99% compared with traditional ILs. To the IL [HMPSM][PF6], the content of imidazolium cation in the aqueous solution before and after extraction with functional IL reduces from 1.24% to 0.85% which means the coordination effect between functional group and the metal ion restrains the release of [HMPSM]+ cation from functional IL to the aqueous solution, while it increases from 0.63% to 0.87% for traditional IL, which is consistent with the results caused by the cation exchange mechanism. In the theory part, the lanl2dz-ECP basis set was employed for transition metal Cu, whereas for the remaining atoms 6-31G (d,p) was applied, cation-anion interaction energies of the ILs and the binding energies between Cu (II) ion and the ILs were calculated, also the surface properties most-negative-surface electrostatic potential (Vs,min) and the lowest surface average local ionization energy (īs,min), were determined by the Multiwfn 2.4 program. All the calculation results show that the sulfur atom from cation is easier to attract the metal ion electrostatically and covalently, thus leads to the high efficiency of extraction.
Key words: thiourea-appended; ionic liquids; extraction; copper; calculation
Liu Mengying , Che Jianing , Wu Weihong , Lu Yunxiang , Peng Changjun , Liu Honglai , Lu Hao , Yang Qiang , Wang Hualin . Extraction of Copper from Aqueous Solution with Functional Ionic Liquids: Experiment and Theoretical Calculation[J]. Acta Chimica Sinica, 2015 , 73(2) : 116 -125 . DOI: 10.6023/A14110779
[1] Kenntner, N.; Krone, O.; Altenkamp, R.; Tataruch, F. Arch. Environ. Contam. Toxicol. 2003, 45, 128.
[2] Swarup, D.; Patre, R. C. Indian J. Anim. Sci. 2005, 75, 231.
[3] Shao, Y.; Ma, Y. Acta Chim. Sinica 2012, 70, 1957. (邵悦, 马勇, 化学学报, 2012, 70, 1957.)
[4] Lemos, A.; Santos, S. Spectrochim. Acta B 2007, 62, 4.
[5] Thakkar, R.; Chudasama, U. J. Hazard. Mater. 2009, 172, 129.
[6] Azeem, S. A.; Arafa, W. A.; El-Shahat, M. F. J. Hazard. Mater. 2010, 182, 286.
[7] Kim, Y. H.; Kim, G. Y.; Lim, H. B. Bull. Korean Chem. Soc. 2010, 31, 908.
[8] Saha, B.; Chakraborty, S.; Das, G. J. Phys. Chem. C 2010, 114, 9817.
[9] Stojanovic, A.; Keppler, B. K. Sep. Sci. Technol. 2012, 47, 189.
[10] Zargoosh, K.; Abedini, H.; Abdolmaleki, A.; Molavian, M. R. Ind. Eng. Chem. Res. 2013, 52, 14944.
[11] Kifle, D.; Wibetoe, G.; Froseth, M.; Bigelius, J. Solvent Extr. Ion Exch. 2013, 3, 1668.
[12] Fan, J.; Fan, Y. C.; Wang, J. J.; Cui, F. L. Acta Chim. Sinica 2006, 64, 1495. (樊静, 范云场, 王键吉, 崔凤灵, 化学学报, 2006, 64, 1495.)
[13] Dietz, M. L. Sep. Sci. Technol. 2006, 41, 2047.
[14] Vidal, S. T.; Correia, M. J.; Marques, M. M.; Ismael, M. R.; Reis, M. T. Sep. Sci. Technol. 2004, 39, 2155.
[15] De los Ríos, A. P.; Lozano, L. J.; Sánchez, S.; Moreno, J.; Godinez, C. J. Chem. Eng. Data 2010, 55, 605.
[16] De los-Ríos, A. P.; Hernandez-Fernandez, F. J.; Alguacil, F. J.; Alguacil, L. J.; Ginestá, A.; García-Díaz, I.; Sánchez-Segado, S.; López, F. A.; Godínez, C. Sep. Sci. Technol. 2012, 97, 150.
[17] Nobuyuki, K.; Yasuhisa, I. Monatsh. Chem. 2007, 138, 1145.
[18] Dai, S.; Ju, Y. H.; Barnes, C. E. J. Chem. Soc. Dalton Trans. 1999, 1201.
[19] Luo, H. M.; Dai, S.; Bonnesen, P. V. Anal. Chem. 2004, 76, 2773.
[20] Luo, H. M.; Dai, S.; Bonnesen, P. V.; Buchanan, A. C.; Holbrey, J. D.; Bridges, N. J.; Rogers, R. D. Anal. Chem. 2004, 76, 3078.
[21] Wei, G. T.; Yang, Z.; Chen, C. J. Anal. Chim. Acta 2003, 488,183.
[22] Li, C. P.; Xin, B. P.; Xu, W. G. J. Dalian Maritime Univ. 2008, 34, 17.
[23] Chen, S.; Sun, H.; Zhong, Y. S. J. Beijing Univ. Technol. 2013, 39, 98. (陈莎, 孙浩, 钟嶷盛, 北京工业大学学报, 2013, 39, 98.)
[24] Hirayama, N.; Deguchic, M.; Kawasumia, H.; Honjo, T. Talanta 2005, 65, 255.
[25] Tsukatani, T.; Katano, H.; Tatsumi, H.; Deguchi , M.; Hirayama, N. Anal. Sci. 2006, 22, 199.
[26] Li, Z. J.; Wei, Q.; Yuan, R.; Zhou, X.; Liu, H. Z.; Shan, H. X.; Song, Q. J. Talanta 2007, 71, 68.
[27] Dadfarnia, S.; Haji-Shabani, A. M.; Bidabadi, M. S.; Ali Jafari, A. J. Hazard. Mater. 2010, 173, 534.
[28] Visser, A. E.; Swatloski, R. P.; Reichert, W. M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J. H.; Rogers, R. D. Environ. Sci. Technol. 2002, 36, 2523.
[29] Papaiconomou, N.; Lee, J. M.; Salminen, J.; Stosch, M.; Prausnitz, J. M. Ind. Eng. Chem. Res. 2008, 47, 5080.
[30] Lee, J. M. Fluid Phase Equilib. 2012, 319, 30.
[31] Ouadi, A.; Gadenne, Hesemann, P.; Moreau-Joel, J. E.; Billard, I.; Gaillard, C.; Mekki, S.; Moutiers, G. Chem. Eur. J. 2006, 12, 3074.
[32] Jitendra, R. H.; Tomislav, F.; Mac-Gillivray, L. R.; Robert, D. S. Inorg. Chem. 2006, 45, 10025.
[33] Fischer, L.; Falta, T.; Koellensperger, G..; Stojanovic, A.; Kogelnig, D.; Galanski, M.; Krachler, R.; Keppler, B. K.; Hann, S. Water Res. 2011, 45, 4601.
[34] Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L. Sep. Sci. Technol. 2013, 107, 172.
[35] Devereux, M.; Popelier, P.; Mclay, I. M. Phys. Chem. Chem. Phys. 2009, 11, 1595.
[36] Bader, R.; Carroll, M. T.; Cheeseman, J. R.; Chang, C. J. Am. Chem. Soc. 1987, 109, 7968.
[37] Bulat, F. A.; Toro-Labbe, A.; Brinck, T.; Murray, J. S.; Politzer, P. J. Mol. Model. 2010, 16, 1679.
[38] Pinter, B.; Nagels, N.; Herrebout, W. A.; De Proft, F. Chem-Eur. J. 2013, 19, 519.
[39] Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049.
[40] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian09 B.01, Gaussian, Inc., Wallingford, CT, 2009.
[41] Wu, W. H.; Lu, Y. X.; Liu, Y. T.; Li, H. Y.; Peng, C. J.; Liu, H. L.; Zhu, W. L. Chem. Phys. Lett. 2013, 582, 49.
[42] Wu, W. H.; Lu, Y. X.; Liu, Y. T.; Peng, C. J.; Liu, H. L. Comput. Theor. Chem. 2014, 1029, 21.
[43] Dong, K.; Song, Y. T.; Liu, X. M.; Cheng, W. G.; Yao, X. Q.; Zhang, S. J. J. Phys. Chem. B 2012, 116, 1007.
[44] Xu, D.; Yang, Q. W.; Su, B. G.; Bao, Z. B.; Ren, Q. L.; Xing, H. B. J. Phys. Chem. B 2014, 118, 1071.
[45] Boys, S. F.; Bernardi, F. Mol. Phys. 1970, 19, 553.
[46] Murray, J. S.; Ranganathan, S.; Politzer, P. J. Org. Chem. 1991, 56, 3734.
[47] Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580.
/
| 〈 |
|
〉 |