

Synthesis and Characterization of Coumarin-containing Hyperbranched-star Copolymers
Received date: 2014-12-30
Online published: 2015-01-28
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21092354, 21374107) and the Fundamental Research Funds for the Central Universities (WK 2060200012).
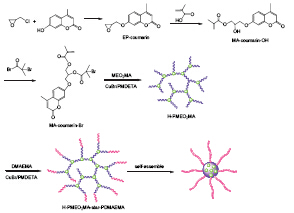
Coumarin-containing inimer was prepared and used in the self-condensing vinyl polymerization (SCVP) of 2-(2-methoxyethoxy)ethyl methacrylate (MEO2MA). The molar ratio of inimer/MEO2MA was set as 1:7, and the reaction was carried out at 50 ℃ for 80 h to afford hyperbranched poly(2-(2-methoxyethoxy)ethyl methacrylate) (H-PMEO2MA). The molecular weight of H-PMEO2MA is 5400 g/mol and Mw/Mn is 1.56. The content of coumarin group in the hyperbranched polymer is consistent with the feed ratio. Then the resultant H-PMEO2MA was used as macroinitiator in the following atom transfer radical polymerization (ATRP) of dimethylaminoethyl methacrylate (DMAEMA), and thermo-responsive hyperbranched-star copolymers with different molecular weights were achieved. NMR and GPC were utilized to characterize the hyperbranched-star copolymers. Lower critical solution temperature (LCST) of H-PMEO2MA is below room temperature, thus the hyperbranched-star copolymers can self-assemble to form micelles in water at room temperature. TEM and DLS were utilized to characterize the morphology and size of the micelles. Coumarin group has the potential to photodimerize via a cycloaddition to form a cyclobutane ring under irradiation of UV light at λ > 310 nm, and the dimer can be reversibly divide into two coumarin groups under irradiation of UV light at λ < 260 nm. In this case, when exposed to irradiation of UV light at λ=320 nm, the micelles were core-crosslinked via photodimerization of the coumarin groups. This procedure has been monitored by the UV-Vis spectrum. With increasing irradiation time, the absorbance of coumarin group at 320 nm decreased. After 30 min, the absorbance almost disappeared, indicating that most of coumarin groups dimerized. Under irradiation at λ=254 nm, de-crosslinking reaction of the crosslinked micelles occurred, with the division of dimer into two coumarin groups. During the self-assembly, Nile Red as the model drug was loaded, and the release of Nile Red under different conditions was investigated. When placed at 25 ℃, both core cross-linked and uncross-linked micelles exhibit a relatively slow release behavior, which suggest the good stability of the micelles. While at 0 ℃, a burst release of the uncross-linked micelles was observed due to the disassembling of micelles. However, the morphology of core cross-linked micelles was kept well, and only a small amount of Nile Red was released.
Key words: coumarin; ATRP; hyperbranched-star polymer; SCVP
Fan Wei , Li Min , Hong Chunyan , Pan Caiyuan . Synthesis and Characterization of Coumarin-containing Hyperbranched-star Copolymers[J]. Acta Chimica Sinica, 2015 , 73(4) : 330 -336 . DOI: 10.6023/A14120897
[1] Kienle, R. H.; Hovey, A. G. J. Am. Chem. Soc. 1929, 51, 509.
[2] Huang, T.; Wang, Z. Y.; Qin, A. J.; Sun, J. Z.; Tang, B. Z. Acta Chim. Sinica 2013, 71, 979 (黄田, 汪昭旸, 秦安军, 孙景志, 唐本忠, 化学学报, 2013, 71, 979.)
[3] Yan, D. Y.; Gao, C. Macromolecules 2000, 33, 7693.
[4] Zhang, L. Y.; Jiao, Q. Z.; Zhao, Y.; Zhou, M. J.; Feng, W.; Ge, Y. R. Acta Chim. Sinica 2011, 69, 2031. (张林雅, 矫庆泽, 赵芸, 周明吉, 冯薇, 葛艳蕊, 化学学报, 2011, 69, 2031.)
[5] You, Y. Z.; Hong, C. Y.; Pan, C. Y.; Wang, P. H. Adv. Mater. 2004, 16, 1953.
[6] Zhou, Y. F.; Yan, D. Y. Chem. Commun. 2009, 10, 1172.
[7] Vogt, A. P.; Sumerlin, B. S. Macromolecules 2008, 41, 7368.
[8] Semsarilar, M.; Ladmiral, V.; Perrier, S. Macromolecules 2010, 43, 1438.
[9] Tao, L.; Chou, W. C.; Tan, B. H.; Davis, T. P. Macromol. Biosci. 2010, 10, 632.
[10] Sheiko, S. S.; Gauthier, M.; Möller, M. Macromolecules 1997, 30, 2343.
[11] Tian, H. Y.; Deng, C.; Lin, H.; Sun, J. R.; Deng, M. X.; Chen, X. S.; Jing, X. B. Biomaterials 2005, 26, 4209.
[12] Lele, B. S.; Leroux, J. C. Polymer 2002, 43, 5595.
[13] Matyjaszewski, K.; Pyun, J.; Gaynor, S. G. Macromol. Rapid Commun. 1998, 19, 665.
[14] Zhang, W. J.; Fan, W.; Li, M.; Hong, C. Y.; Pan, C.Y. Acta Chim. Sinica 2012, 70, 1690. (张文建, 范溦, 李敏, 洪春雁, 潘才元, 化学学报, 2012, 70, 1690.)
[15] Shuai, X. T.; Merdan, T.; Schaper, A. K.; Xi, F.; Kissel, T. Bioconjugate Chem. 2004, 15, 441.
[16] Kakizawa, Y.; Harada, A.; Kataoka, K. Biomacromolecules 2001, 2, 491.
[17] Li, Y. L. Ph.D. Dissertation, Soochow University, Suzhou, 2010. (李玉玲, 博士论文, 苏州大学, 苏州, 2010.)
[18] Trenor, S. R.; Shultz, A. R.; Love, B. J.; Long, T. E. Chem. Rev. 2004, 104, 3059.
[19] Ling, J.; Rong, M. Z.; Zhang, M. Q. Chin. J. Polym. Sci. 2014, 32, 1286.
[20] Mal, N. K.; Fujiwara, M.; Tanaka, Y. Nature 2003, 421, 350.
[21] Jiang, J. Q.; Qi, B.; Lepage, M.; Zhao, Y. Macromolecules 2007, 40, 790.
[22] Jia, Z. F.; Zhou, Y. F.; Yan, D. Y. J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 6534.
[23] Zhang, K. J.; Wang, J. L.; Subramanian, R.; Ye, Z. B.; Lu, J. M.; Yu, Q. Macromol. Rapid Commun. 2007, 28, 2185.
[24] Muthukrishnan, S.; Mori, H.; Müller, A. H. E. Macromolecules 2005, 38, 3108.
[25] Muthukrishnan, S.; Jultz, G.; André, X.; Mori, H.; Müller, A. H. E. Macromolecules 2005, 38, 9.
[26] Rikkou-Kalourkoti, M.; Matyjaszewski, K.; Patrickios, C. S. Macromolecules 2012, 45, 1313.
[27] Luzon, M.; Boyer, C.; Peinado, C.; Corrales, T.; Whittaker, M.; Tao, L.; Davis, T. J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 2783.
[28] Gillies, E. R.; Jonsson, T. B.; Fréchet, J. M. J. J. Am. Chem. Soc. 2004, 126, 11936.
[30] Jiang, X. G.; Zhao, B. Macromolecules 2008, 41, 9366.
/
| 〈 |
|
〉 |