

Effect of Mesoporous TiO2 Layer Thickness on the Cell Performance of Perovskite Solar Cells
Received date: 2014-11-29
Online published: 2015-02-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 91433205, 51421002).
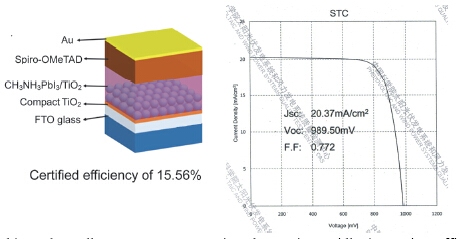
Perovskite solar cells attract great attention due to its rapidly increasing efficiency. Conventional structure of perovskite solar cell contains FTO glass substrate, compact TiO2 layer, mesoporous TiO2/CH3NH3PbI3 layer, hole transporting material layer and Au counter electrode. In this work, we fabricated perovskite solar cells with the above conventional structure. The mesoporous TiO2 layer thickness are 500, 350, 150 and 100 nm. Thickness of CH3NH3PbI3 capping layer is about 300 nm. The perovskite films and solar cells were characterized by SEM, XRD, UV-Vis absorption spectrum, photocurrent-photovoltage characteristics and electrochemical impedance spectra. XRD patterns of the perovskite films are similar, indicating the complete transfer from PbI2 to CH3NH3PbI3. Statistical results of short-circuit current, open-circuit voltage, fill factor and power conversion efficiency are compared, revealing that as mesoporous TiO2 layer thickness increasing, both photovoltage and fill factor decrease whereas short-circuit current slightly increases. Solar cells with thinner mesoporous TiO2 layer can give higher efficiency. Besides, the devices with 100 and 150 nm mesoporous TiO2 layers can present the average efficiency of 15%. The decrement of efficiency is supposed to be caused by stronger carrier recombination. Electrochemical impedance spectra and current-voltage characteristics under dark condition were applied to characterize the carrier recombination process. Nyquist plots demonstrated an increment of the recombination as the mesoporous TiO2 layer thickness increases. Charge transfer resistances were obtained by fitting Nyquist plots. The charge transfer resistances of solar cells with 100 and 350 nm mesoporous TiO2 layer decrease with bias voltage exponentially in similar slope, indicating that this change of recombination do not influence the diode quality factor. Reverse saturated current density was obtained by fitting dark current-voltage curves. The reverse saturated current densities have positive correction with mesoporous TiO2 layer thickness. As a conclusion, the change of the recombination is caused by reverse saturated current density rather than diode quality factor. Further investigation revealed that the devices with thinner mesoporous TiO2 layers exhibit relatively stronger hysteresis behavior. 15.56% of certified efficiency has been obtained for the perovskite solar cell with 150 nm-thickness mesoporous TiO2 layer.
Key words: perovskite; solar cell; carrier recombination; TiO2; film thickness
Zhu Lifeng , Shi Jiangjian , Li Dongmei , Meng Qingbo . Effect of Mesoporous TiO2 Layer Thickness on the Cell Performance of Perovskite Solar Cells[J]. Acta Chimica Sinica, 2015 , 73(3) : 261 -266 . DOI: 10.6023/A14110823
[1] (a) Kazim, S.; Nazeeruddin, M. K.; Grätzel, M.; Ahmad, S. Angew. Chem., Int. Ed. 2014, 53, 2812;
(b) Snaith, H. J. J. Phys. Chem. Lett. 2013, 3623.
[2] Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. J. Am. Chem. Soc. 2009, 131, 6050.
[3] Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J. E.; Graetzel, M.; Park, N.-G. Sci. Rep. 2012, 2, 591.
[4] (a) http://www.nrel.gov/ncpv/images/efficiency_chart.jpg;
(b) Park, N.-G. J. Phy. Chem. Lett. 2013, 4, 2423;
(c) Guo, X.; Niu, G.; Wang, L. Acta Chim. Sinica 2015, 73, 211. (郭旭东, 牛广达, 王立铎, 化学学报, 2015, 73, 211.);
(d) Zhang, D.; Zheng, L.; Ma, Y.; Wang, S.; Bian, Z.; Huang, C.; Gong, Q.; Xiao, L. Acta Phys. Sin. 2014, 64, 038803. (张丹霏, 郑灵灵, 马英壮, 王树峰, 卞祖强, 黄春辉, 龚旗煌, 肖立新, 物理学报, 2014, 64, 038803.)
[5] (a) Liu, M.; Johnston, M. B.; Snaith, H. J. Nature 2013, 501, 395;
(b) Eperon, G. E.; Burlakov, V. M.; Docampo, P.; Goriely, A.; Snaith, H. J. Adv. Funct. Mater. 2014, 24, 151.
[6] (a) Abu Laban, W.; Etgar, L. Energy Environ. Sci. 2013, 6, 3249;
(b) Yang, Y.; Xiao, J.; Wei, H.; Zhu, L.; Li, D.; Luo, Y.; Wu, H.; Meng, Q. RSC Adv. 2014, 4, 52825.
[7] Seo, J.; Park, S.; Kim, Y. C.; Jeon, N. J.; Noh, J. H.; Yoon, S. C.; Seok, S. I. Energy Environ. Sci. 2014, 7, 2642.
[8] Lee, M. M.; Teuscher, J.; Miyasaka, T.; Murakami, T. N.; Snaith, H. J. Science 2012, 338, 643.
[9] Xue, Q.; Sun, C.; Hu, Z.; Huang, F.; Yip, H.-L.; Cao, Y. Acta Chim. Sinica 2015, 73, 179. (薛启帆, 孙辰, 胡志诚, 黄飞, 叶轩立, 曹镛, 化学学报, 2015, 73, 179.)
[10] Fabregat-Santiago, F.; Garcia-Belmonte, G.; Mora-Sero, I.; Bisquert, J. Phys. Chem. Chem. Phys. 2011, 13, 9083.
[11] (a) Dualeh, A.; Moehl, T.; Tetreault, N.; Teuscher, J.; Gao, P.; Nazeeruddin, M. K.; Graetzel, M. ACS Nano 2014, 8, 362;
(b) Kim, H.-S.; Mora-Sero, I.; Gonzalez-Pedro, V.; Fabregat-Santiago, F.; Juarez-Perez, E. J.; Park, N.-G.; Bisquert, J. Nat. Commun. 2013, 4, 2242.
[12] Christians, J. A.; Fung, R. C. M.; Kamat, P. V. J. Am. Chem. Soc. 2014, 136, 758.
[13] Shi, J.; Dong, J.; Lv, S.; Xu, Y.; Zhu, L.; Xiao, J.; Xu, X.; Wu, H.; Li, D.; Luo, Y.; Meng, Q. Appl. Phys. Lett. 2014, 104, 063901.
[14] Xu, Y.; Shi, J.; Lv, S.; Zhu, L.; Dong, J.; Wu, H.; Xiao, Y.; Luo, Y.; Wang, S.; Li, D. Meng, Q. ACS Appl. Mater. Interfaces 2014, 6, 5651.
[15] Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M. K.; Gratzel, M. Nature 2013, 499, 316.
/
| 〈 |
|
〉 |