

Effects of Organic Additives Containing Carbonyl Group on Electrodeposition of Al from AlCl3-[Emim]Cl Ionic Liquid
Received date: 2014-12-12
Online published: 2015-04-01
Supported by
Project supported by the National Key Basic Research Program of China (No. 2015CB251401), the Key Technologies R&D Program (No. 2012BAF03B01), the Special Funds of the National Natural Science Foundation of China (No. 21127011) and the General Program of National Natural Science Foundation of China (No. 51274181).
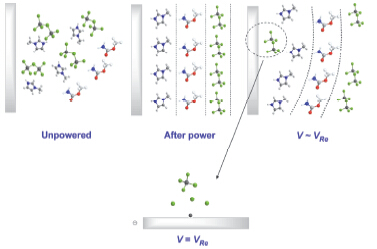
Electrodeposition of Al in ionic liquids for preparation of aluminum products had opened up a new research direction, but the previous studies found that aluminum product from pure ionic liquid electrodeposition was unable to meet the requirements in both uniformity and smoothness. Electrodepostion in pure ionic liquid often results in the deposits with rough surface or even dendritic products after long-time deposition. In this study, five kinds of small organic molecules containing carbonyl as additives were investigated, such as acetone, acetic acid amide, acetic acid, methyl acetate and methyl carbamate. Additives were added to [Emim]Cl/AlCl3(33.3/66.7 mol%) with a concentration of 5 mmol/L, 15 mmol/L, 45 mmol/L. Cyclic voltammetry was studied on a glassy carbon electrode at a scan rate of 100 mV·s-1 and temperature of 323 K. The electrode potential was scanned from the open circuit potential to negative-potential, then retraced to get cyclic voltammetry curve. Among those organic additives, methyl carbamate was proved to be an excellent additive. The brightness of Al can be improved by adding 45 mmol/L methyl carbamate. Smooth, uniform and mirror bright Al deposition would be obtained afterward. Furthermore, the effects of additive for Al deposit on morphology, crystal orientation and Al deposition mechanism by the analysis of galvanostatic deposition, scanning electron microscope (SEM), X-ray diffraction (XRD), ultraviolet-visible spectroscopy (UV-Vis) and nuclear magnetic resonance (NMR) was conjectured. The results showed that methyl carbamate as an additive would make grain refinement more apparent and present more strong Al(200) crystal plane orientation. As an additive, methyl carbamate did not form new metal complex in the electrolytic liquid and did not affect the structure of active aluminum ion in the electrolyte. The positive center of carbonyl carbon atom in methyl carbamate molecular can be easily adsorbed at the cathode surface. This procedure inhibited the process of Al deposition and obtained the smooth and bright effect. Therefore, Al deposition layer of mirror can be obtained, which has an important guiding significance in the selection of ionic liquid additive in electrodeposition system.
Key words: ionic liquids; electrodeposition; carbonyl; methyl carbamate; additives
Leng Minghao , Chen Shimou , Zhang Junling , Lang Haiyan , Kang Yanhong , Zhang Suojiang . Effects of Organic Additives Containing Carbonyl Group on Electrodeposition of Al from AlCl3-[Emim]Cl Ionic Liquid[J]. Acta Chimica Sinica, 2015 , 73(5) : 403 -408 . DOI: 10.6023/A14120857
[1] Chang, J. K.; Chen, S. Y.; Tsai, W. T.; Deng, M. J.; Sun, I. W. Electrochem. Commun. 2007, 9(7), 1602.
[2] Endo, A.; Miyake, M.; Hirato, T. Electrochim. Acta 2014, 137, 470.
[3] NuLi, Y. N.; Du, G. D.; Feng, Z. Z.; Shen, J.-N.; Yang, J. Acta Chim. Sinica 2008, 66, 175. (努丽燕娜, 杜国栋, 冯真真, 沈佳妮, 杨军, 化学学报, 2008, 66, 175.)
[4] NuLi, Y. N.; Yang, J.; Gao, P. F.; Li, Y.; Wang, J. L. Acta Chim. Sinica 2010, 68, 948. (努丽燕娜, 杨军, 高鹏飞, 李云, 王久林, 化学学报, 2010, 68, 948.)
[5] Hurley, F. H.; Wier, T. P. J. Electrochem. Soc. 1951, 85(5), 207.
[6] Liao, Q.; Pitner, W. R.; Stewart, G.; Hussey, C. L.; Stafford, G. R. J. Electrochem. Soc.1997, 144(3), 936.
[7] Lee, J. J.; Bae, I. T.; Scherson, D. A.; Miller, B.; Wheeler, K. A. J. Electrochem. Soc.2000, 147(2), 562.
[8] Liu, Q. X.; El. Abedin, S. Z.; Endres, F. Surf. Coat. Technol. 2006, 201, 1352.
[9] Tang, J. W.; Azumi, K. Electrochim. Acta 2011, 56, 1130.
[10] Zhao, Y. G.; VanderNoot, T. J. Electrochim. Acta 1997, 42(11), 1639.
[11] Chang, J. K.; Chen, S. Y.; Tsaia, W. T.; Deng, M. J.; Sun, I. W. J. Electrochem. Soc.2008, 155(3), C112.
[12] Yue, G. K.; Zhang, S. J.; Zhu, Y. L.; Lu, X. M.; Li, S. C.; Li, Z. X. AIChE J. 2009, 55, 783.
[13] Jiang, T.; Brym, M. J. C.; Dube, G.; Lasia, A.; Brisard, G. M. Surf. Coat. Technol.2006, 201, 10.
[14] Su, C. J.; Hsieh, Y. T.; Chen, C. C. Electrochem. Commun. 2013, 34, 170.
[15] El. Abedin, S. Z.; Moustafa, E. M.; Hempelmann, R.; Natter, H.; Endres, F. Electrochem. Commun. 2005, 7, 1111.
[16] El. Abedin, S. Z.; Moustafa, E. M.; Hempelmann, R.; Natter, H.; Endres, F. ChemPhysChem 2006, 7(11), 1535.
[17] Moustafa, E. M.; El. Abedin, S. Z.; Shkurankov, A.; Zschippang, E.; Saad, A. Y.; Bund, A.; Endres, F. J. Phys. Chem. B 2007, 111, 4693.
[18] Atkin, R.; El. Abedin, S. Z.; Hayes, R.; Gasparotto, L. H. S.; Borisenko, N.; Endres, F. J. Phys. Chem. C 2009, 113, 13266.
[19] Giridhar, P.; El. Abedin, S. Z.; Endres, F. Electrochim. Acta 2012, 70, 21.
[20] Zheng, Y.; Dong, K.; Wang, Q.; Zhang, S. J.; Zhang, Q. Q.; Lu, X. M. Sci. China Chem. 2012, 55(8), 1587.
[21] Zheng, Y.; Zhang, S. J.; Lu, X. M.; Wang, Q.; Zuo, Y.; Liu, L. Chinese J. Chem. Eng.2012, 20(1), 130.
[22] Abbott, A. P.; Qiu, F.; Abood, H. M. A.; Ali, M. R.; Ryder, K. S. Phys. Chem. Chem. Phys. 2010, 12, 1862.
[23] Liu, L.; Lu, X. M.; Cai, Y. J.; Zheng, Y.; Zhang, S. Z. Aust. J. Chem. 2012, 65(11), 1523.
[24] Li, B.; Fan, C. H.; Chen, Y.; Lou, J. W.; Yan, L. G. Electrochim. Acta 2011, 56(16), 5478.
[25] Barchi, L.; Bardi, U.; Caporali, S.; Fantini, M.; Scrivani, A. Prog. Org. Coat. 2010, 68, 120.
[26] Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Angew. Chem. Int. Ed. 2003, 42, 3428.
[27] Zhang, Q. Q.; Wang, Q.; Zhang, S. Z.; Lu, X. M. J. Solid State Electrochem. 2014, 18(1), 257.
[28] Wang, X. M. Ph.D. Dissertation Shandong University of Technology, Zibo, 2010. (王晓铭, 博士论文, 山东理工大学, 淄博, 2010.)
[29] Zhu, Y. L.; Katayama, Y.; Miura, T. Electrochim. Acta 2012, 85(4), 622.
[30] Zhu, Y. L.; Katayama, Y.; Miura, T. Electrochim. Acta 2010, 55(28), 9019.
/
| 〈 |
|
〉 |