

Chemical Constituents with Neuraminidase Inhibitory Actives from Campylotropis Hirtella
Received date: 2015-03-26
Online published: 2015-04-03
Supported by
Supporting information for this article is available free of charge via the Internet at http://sioc-journal.cn.Project supported by the Science & Technology commission of Shanghai municipal government (No.14401900600).
Influenza is and remains a disease to reckon with. Seasonal epidemics around the world kill several hundred thousand people every year. Development of effective drugs for the treatment or prevention of epidemic and pandemic influenza is important in order to reduce its impact. Neuraminidase inhibitors is a class of anti-influenza drugs available for influenza therapy currently. However, emergence of resistance to these drugs has been detected, which raises concerns regarding their widespread use. Therefore, the search for low toxic natural neuraminidase inhibitor is one of the route for finding new anti-influenza virus drugs. In current study, the classical MUNANA-based NA activity assay was established to assess the neuraminidase inhibitory activities of compounds isolated from Campylotropis hirtella. The bioassay guided separation of 95% ethanol extract of the dried root of C. hirtella has led to the isolation of ten compounds, and their structures were elucidated on the basis of spectroscopic methods, with special emphasis on 1D and 2D NMR techniques. Compounds 1~4 are new compounds and these are two chromones, an isoflavone and a glycosides. Compounds 5~10 were isolated from C. hirtella for the first time. All compounds were assesses for their inhibitory activities against influenza A1(H1N1) virus NA. Compounds 1, 2, 3, 5 showed good and dose dependent inhibitory effects against NA enzyme. Among them, compound 1 had the best NA inhibitory activity with the IC50 at 16.76 μmol/L. Although, compound 1 is less active than Tamiflu, however, the results from the study demonstrated that isoflavone and chromone could be potent neuraminidase inhibitor. Since the structure of compound 1 is very similar to Hirtellanine B, an isoflavonoid isolated from the same herb with potent antiproliferative effects in cancer cells, the biosynthetic pathway of both compound 1 and Hirtellanine B were proposed.
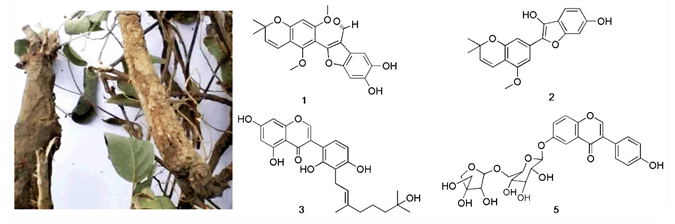
Du Xin , Xuan Bixia , Shen Zhengwu . Chemical Constituents with Neuraminidase Inhibitory Actives from Campylotropis Hirtella[J]. Acta Chimica Sinica, 2015 , 73(7) : 741 -748 . DOI: 10.6023/A15030209
[1] Alexander, D. J.; Brown, I. H. Rev. Sci. Tech. Off. Int. Epiz. 2000, 19, 197.
[2] Juozapaitis, M.; Antoniukas, L. Medicina (Kaunas) 2007, 43, 919.
[3] Hurt, A. C.; Lee, R. T.; Leang, S. K.; Cui, L.; Deng, Y. M.; Phuah, S. P.; Caldwell, N.; Freeman, K.; Komadina, N.; Smith, D. Euro Surveill. 2011, 16, 19884.
[4] Ryu, Y. B.; Curtis-Long, M. J.; Kim, J. H.; Jeong, S. H.; Yang, M. S.; Lee, K. W.; Lee, W. S.; Park, K. H. Bioorg. Med. Chem. Lett. 2008, 18, 6046.
[5] China Pharmacopoeia Committee, Chinese Pharmacopoeia, Beijing, 1978, p. 30. (中华人民共和国卫生部药典委员会, 中华人民共和国药典, 北京, 1978, p. 30.)
[6] Zhang, S.; Tan, Q.; Shou, Q.-Y. Acta Chim. Sinica 2010, 68, 2227. (张盛, 谭青, 寿清耀, 化学学报, 2010, 68, 2227.)
[7] Shou, Q.-Y.; Tan, Q.; Shen, Z-.W. Bioorg. Med. Chem. Lett. 2009, 19, 3389.
[8] Shou, Q.-Y.; Tan, Q.; Shen, Z.-W. Planta Med. 2009, 75, 1.
[9] Shou, Q.-Y.; Tan, Q.; Shen, Z.-W. J. Agric. Food Chem. 2009, 57, 6712.
[10] Tan, Q.; Zhang, S.; Shen, Z.-W. Planta Med. 2011, 77, 1811.
[11] Li, X.-P.; Xuan, B.-X.; Shen, Z.-W. Fitoterapia 2014, 95, 220.
[12] Han, H.-Y.; Wen, P.; Liu, H.-W.; Wang, N.-L.; Yao, X.-S. Chem. Pharm. Bull. 2008, 56, 1338.
[13] Han, H.-Y.; Wang, X.-H.; Wang, N.-L.; Ling, M. T.; Wong, Y. C.; Yao, X. S. J. Agric. Food Chem. 2008, 56, 6928.
[14] Xiao, C.-H. Chemistry of Chinese Materia Medica, Shanghai Science and Technology Press, Shanghai, 1987, p. 165. (肖崇厚, 中药化学, 上海科学技术出版社, 上海, 1987, p. 165.)
[15] Cui, X.-Q.; Wang, L.; Yan, R.-Y.; Tan, Y.-X.; Chen, R.-Y.; Yu, D.-Q. J. Asian Nat. Prod. Res. 2008, 10, 315.
[16] Xia, Z.-T.; Liu, D.-Y.; Wang, X.-Y. Acta Chim. Sinica 2004, 62, 1935. (夏忠庭, 刘大有, 王晓颖, 化学学报, 2004, 62, 1935.)
[17] Qin, X.-J.; Lunga, P. K.; Zhao, Y.-L.; Li, J.-L.; Yang, X.-W.; Liu, Y.-P.; Luo, X.-D. Fitoterapia 2014, 92, 238.
[18] Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Phytochemistry 2002, 59, 565.
[19] Ma, W.-G.; Fukushi, Y.; Hostettmann, K.; Tahara, S. Phytochemistry 1998, 49, 251.
[20] Bao, G.-H.; Wang, L.-Q.; Cheng, K.-F.; Feng, Y.-H.; Li, X.-Y.; Qin, G.-W. Planta Med. 2003, 69, 434.
[21] El-Sayed, N. H.; Wojcinska, M.; Drost-Karbowska, K.; Matlawska, I.; Williams, J.; Mabry, T. J. Phytochemistry 2002, 60, 835.
[22] Djimtombaye, B. J.; Alankus-Caliskan, O.; Gulcemal, D.; Khan, I. A.; Anil, H.; Bedir, E. Chem. Biodiversity 2013, 10, 1328.
[22] Wu, X.-D.; Wang, L.; He, J.; Li, X.-Y.; Dong, L.-B.; Gong, X.; Gao, X.; Song, L.-D.; Li, Y.; Peng, L.-Y.; Zhao, Q.-S. Helv. Chim. Acta 2013, 96, 2207.
/
| 〈 |
|
〉 |