

Synthesis and Properties Study of Novel Branched Fluorinated Surfactants with CF3CF2CF2C(CF3)2 Group
Received date: 2015-03-13
Online published: 2015-04-08
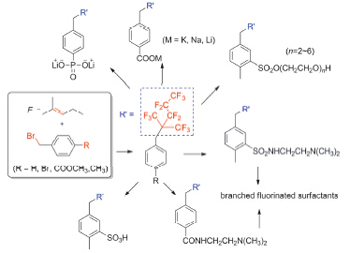
Perfluorooctanoic acid (PFOA) and perfluorooctane sulphonate (PFOS) were listed under Stockholm convention on persistent organic pollutants owing to their poor biodegradability and toxic effects in the environment, so various strategies were reported for synthesizing non-bioaccumulable alternatives to them. Herein, we described our recent strategies for synthesis of non-bioaccumulable fluorinated surfactants by introducing branched perfluorinated chain using perfluoro-2-methyl-2-pentene as starting material. Novel branched fluorinated cationic, gemini and amphoteric surfactants were designed and prepared through a four-step route. They all have low solubility in water and exhibit poor ability to reduce the surface tension of water. A series of branched fluorinated nonionic surfactants with different length of hydrophilic chains were synthesized via a three-step route. The solubility becomes larger with the increase of the value of polymerization degree of polyethylene glycol. But, the surface tension measurement results show that their ability and efficiency to reduce the surface tension of water change little when the value of polymerization degree of polyethylene glycol is equal to or greater than 4. All the values of surface activities of the novel 20~22 surfactants (take the compound 20 for example, about 21.4 mN/m at the cmc 4.4×10-5 mol/L) are lower than that of sodium perfluorooctanoate (about 24.7 mN/m at the cmc 3.1×10-2 mol/L). Methyl-benzenesulfonic acid (compound 23) was synthesized through a two-step route. Both the steps are easy work-up, mild reaction conditions, low cost and high yields. The surfactant exhibits excellent ability to reduce the surface tension of water to 20.8 mN/m. Besides, it has good salt-resistance. The acute toxicity of 20 was investigated and the result shows that the acute oral toxicity LD50 Value of the compound 20 was 3160 mg/kg which is higher than that of ammonium perfluorooctanoate (C7F15COONH4, 470~540 mg/kg). So preparing branched fluorinated surfactants using hexafluoropropylene (HFPD) is a simple, economical and environmentally friendly method for synthesis of alternatives to PFOA and PFOS.
Sha Min , Zhang Ding , Pan Renming , Xing Ping , Jiang Biao . Synthesis and Properties Study of Novel Branched Fluorinated Surfactants with CF3CF2CF2C(CF3)2 Group[J]. Acta Chimica Sinica, 2015 , 73(5) : 395 -402 . DOI: 10.6023/A15030174
[1] Song, A.-X.; Dong, S.-L.; Hao, J.-C.; Liu, W.-M.; Xu, G.-Y.; Wang, H.-Q. J. Fluorine Chem. 2005, 126, 1266.
[2] Lu, C. Q.; Kim, J. H.; DesMarteau, D. D. J. Fluorine Chem. 2010, 131, 17.
[3] Gramstad, T. R.; Haszeldine, N. J. Chem. Soc. 1957, 4069.
[4] Krafft, M. P.; Riess, J. G. J. Polym. Sci., Part A: Polym. Chem. 2007, 45, 1185.
[5] Kaplánek, R.; Paleta, O.; Ferjentsiková, I.; Kodícek, M. J. Fluorine Chem. 2009, 130, 308.
[6] Caillier, L.; Taffin de Givenchy, E.; Levy, R.; Vandenberghe, Y.; Geribaldi, S.; Guittard, F. J. Colloid Interface Sci. 2009, 332, 201.
[7] Prescher, D.; Gross, U.; Wotzka, J.; Txchen-Schlueter, M.; Starke, W. Acta Hydrochim. Hydrobiol. 1985, 13, 17.
[8] Key, B. D.; Howell, R. D.; Criddle, C. S. Environ. Sci. Technol. 1997, 31, 2445.
[9] Key, B. D.; Howell, R. D.; Criddle, C. S. Environ. Sci. Technol. 1998, 32, 2283.
[10] Houde, M.; Martin, J. W.; Letcher, R. J.; Solomon, K. R.; Muir, D. C. G. Environ. Sci. Technol. 2006, 40, 3463.
[11] U.S. Environmental Protection Agency, http://www.epa.gov/ oppt/pfoa/pubs/stewardship/index.html.
[12] Drakesmith, F. G.; Hughes, D. A. J. Appl. Electrochem. 1979, 9, 685.
[13] Haszeldine, R. N. Nature 1951, 167, 139.
[14] Haszeldine, R. N. J. Chem. Soc. 1949, 2856.
[15] Murphy, P. M.; Baldwin, C. S.; Buck, R. C. J. Fluorine Chem. 2012, 138, 3.
[16] Boutevin, G.; Tiffes, D.; Loubat, C. J. Fluorine Chem. 2012, 134, 7.
[17] Dmowski, W.; Plenkiewicz, H.; Piasecka-Maciejewska, K.; Prescher, D.; Schulze, J.; Endler, I. J. Fluorine Chem. 1990, 48, 77.
[18] Liu, Z.-M.; Wu, J.-F.; Tan, L.-M.; Hu, Y.-M.; Song, Z.-C.; Yu, X.-X. Fine Chem. 2005, 22, 53. (刘在美, 吴京峰, 谈龙妹, 胡应模, 宋志超, 俞雪兴, 精细化工, 2005, 22, 53.)
[19] Yang, B.-Q.; Wang, L.; Yang, J.; Liu, J. Chem. Res. Appl. 2013, 25, 1523. (杨百勤, 王林, 杨健, 刘瑾, 化学研究与应用, 2013, 25, 1523.)
[20] Zheng, S.-C.; Zhao, Y.-J.; Lei, Z.-G.; Zhou, Q.; Wang, S.-H. Chem. Prod. Technol. 2012, 19, 6. (郑士才, 赵颖俊, 雷志刚, 周强, 王树华, 化工生产与技术, 2012, 19, 6.)
[21] Qi, H.; Xu, W.-G.; Zhao, W.-J.; Chen, W.; Liu, Y.-L.; Zhang, Y.-Y. Organo-Fluorine Industry 2012, 4, 18. (齐海, 徐卫国, 赵卫娟, 陈伟, 刘毓林, 张勇耀, 有机氟化工, 2012, 4, 18.)
[22] Xu, J.-H.; Chen, Z.-J.; Zhang, W.-B. Organo-Fluorine Industry 2007, 3, 29. (徐金和, 陈志军, 张巍彪, 有机氟化工, 2007, 3, 29.)
[23] Ikeda, I.; Tsuji, M.; Okahara, M. Tenside, Surfactants, Detergents 1987, 24, 272.
[24] Tomota, H.; Nakayama, N. JP 03093744, 1991 [Chem. Abstr. 1991, 115, 231866].
[25] Koga, K.; Nemoto, F. JP 61069754, 1986 [Chem. Abstr. 1986, 105, 133514].
[26] Zhou, H.-T.; Gao, A.-T.; Xing, H.; Gou, Z.-M.; Xiao, J.-X. Acta Chim. Sinica 2011, 69, 1035. (周洪涛, 高岸涛, 邢航, 勾志明, 肖进新, 化学学报, 2011, 69, 1035.)
[27] Wang, C.; Chen, X.-Y.; Zhu, Z.; Xiao, J.-X. Acta Chim. Sinica 2009, 67, 1425. (王晨, 陈新远, 朱湛, 肖进新, 化学学报, 2009, 67, 1425.)
[28] Xing, H.; Lin, C.-X.; Xiao, J.-X. Acta Chim. Sinica 2008, 66, 1382. (邢航, 林崇熙, 肖进新, 化学学报, 2008, 66, 1382.)
[29] Jin, C.; Yan, P.; Wang, C.; Xiao, J.-X. Acta Chim. Sinica 2005, 63, 279. (金辰, 严鹏, 王晨, 肖进新, 化学学报, 2005, 63, 279.)
[30] Sha, M.; Pan, R.-M.; Zhan, L.-W.; Xing, P.; Jiang, B. Chin. J. Chem. 2014, 32, 995.
[31] Sha, M.; Pan, R.-M.; Xing, P.; Jiang, B. J. Fluorine Chem. 2015, 169, 61.
[32] Zhang, Q.-S.; Luo, Z.-Y.; Curran, D. P. J. Org. Chem. 2000, 65, 8866.
/
| 〈 |
|
〉 |