

Synthesis and Aggregation-induced Emission of Tetraphenylethylene Derivatives Based on Hexakisphenylbenzene Backbone
Received date: 2015-04-02
Online published: 2015-04-30
Supported by
Project supported by the 973 Program (Nos. 2013CB834703, 2013CB834505) and the National Natural Science Foundation of China (Nos. 21273258, 21233011, 21172229, 21004072).
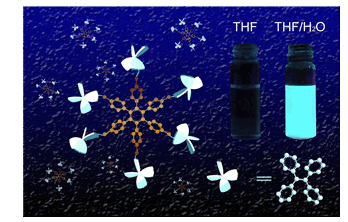
A series of tetraphenylethylene (TPE) derivatives based on hexakisphenylbenzene backbone (HPB-nTPE, n=2, 4, 6) were synthesized by cobalt carbonyl-catalyzed cyclotrimerization of alkynes with 1,2-bis(4-(tert-butyl)phenyl)ethyne and 1,2-bis(4'-(1,2,2-triphenylvinyl)-[1,1'-biphenyl]-4-yl)ethyne (TPE-TPE) as starting materials. By varying the ratio of the starting materials, HPB-nTPE (n=2, 4, 6) with 2, 4 or 6 TPE substituents were obtained. The structures of HPB-nTPE (n=2, 4, 6) were characterized by IR, 1H NMR and MALDI-TOF mass spectra. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) show that the decomposition temperatures of HPB-nTPE are all above 440 ℃, indicative of high thermal stability of HPB-nTPE. The photophysical properties of the tetrahydrofuran solution, the aggregate and the neat solid of HPB-nTPE were investigated. The aggregates of HPB-nTPE (n=2, 4, 6) were obtained in tetrahydrofuran/water mixed solvents with various volume fractions of water, which was confirmed by the scanning electron microscope (SEM). HPB-nTPE (n=2, 4, 6) show typical aggregation induced emission properties, almost non-emissive in tetrahydrofuran solution, but strongly emissive in the aggregate or solid state. The highest fluorescence quantum yields for HPB-nTPE (n=2, 4, 6) in aggregate state are 0.37, 0.36 and 0.37, respectively, which are more than 400 times higher than that in tetrahydrofuran. The quantum yields of the neat solid of HPB-nTPE (n=2, 4, 6) are determined to be 0.39, 0.36 and 0.36, which are ca. 1.8 times of that of the neat solid of tetraphenylethylene. Both restrictions of the intramolecular rotation of phenyl rings and the inter/intramolecular π-π stacking of the TPE chromophores caused by the star-shape rigidity structure of hexakisphenylbenzene suppress the nonradiative transition, consequently, giving higher fluorescence quantum yield of the aggregates and the neat solid of HPB-nTPE in comparison with TPE. This work is of importance for developing highly emissive organic materials in solid states.
Xun Zhiqing , Tang Haiyun , Zeng Yi , Chen Jinping , Yu Tianjun , Zhang Xiaohui , Li Yi . Synthesis and Aggregation-induced Emission of Tetraphenylethylene Derivatives Based on Hexakisphenylbenzene Backbone[J]. Acta Chimica Sinica, 2015 , 73(8) : 819 -825 . DOI: 10.6023/A15040224
[1] (a) Maggini, L.; Bonifazi, D. Chem. Soc. Rev. 2012, 41, 211;
(b) Bonacchi, S.; Genovese, D.; Juris, R.; Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Angew. Chem., Int. Ed. 2011, 50, 4056;
(c) Wang, N.; Si, J.; Jin, Y.; Wang, J.; Huang, W. Acta Chim. Sinica 2015, 73, 171. (王娜娜, 司俊杰, 金一政, 王建浦, 黄维, 化学学报, 2015, 73, 171.);
(d) Wang, F.; Tao, Y.; Huang, W. Acta Chim. Sinica 2015, 73, 9. (王芳芳, 陶友田, 黄维, 化学学报, 2015, 73, 9.);
(e) Xu, G.; Li, J.; Chen, Z. Acta Chim. Sinica 2014, 72, 667. (徐广涛, 李佳, 陈忠宁, 化学学报, 2014, 72, 667.);
(f) Li, J.; Zeng, Y.; Zhang, X.; Yu, T.; Chen, J. P.; Li, Y. Acta Chim. Sinica 2014, 72, 1157. (李婧, 曾毅, 张小辉, 于天君, 陈金平, 李嫕, 化学学报, 2014, 72, 1157.)
[2] Jakubiak, R.; Collison, C. J.; Wan, W. C.; Rothberg, L. J.; Hsieh, B. R. J. Phys. Chem. A 1999, 103, 2394.
[3] (a) Mukherjee, S.; Thilagar, P. Dyes and Pigments 2014, 110, 2;
(b) Kalyani, N. T.; Dhoble, S. J. Renew. Sust. Energ. Rev. 2012, 16, 2696.
[4] Luo, J. D.; Xie, Z. L.; Lam, J. W. Y.; Cheng, L.; Chen, H. Y.; Qiu, C. F.; Kwok, H. S.; Zhan, X. W.; Liu, Y. Q.; Zhu, D. B.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
[5] (a) Chen, J.; Xie, Z.; Lam, J. W. Y.; Law, C. C. W.; Tang, B. Z. Macromolecules 2003, 36, 1108;
(b) Chen, J. W.; Law, C. C. W.; Lam, J. W. Y.; Dong, Y. P.; Lo, S. M. F.; Williams, I. D.; Zhu, D. B.; Tang, B. Z. Chem. Mater. 2003, 15, 1535.
[6] (a) Mei, J.; Hong, Y. N.; Lam, J. W. Y.; Qin, A. J.; Tang, Y. H.; Tang, B. Z. Adv. Mater. 2014, 26, 5429;
(b) Hu, R.; Leung, N. L. C.; Tang, B. Z. Chem. Soc. Rev. 2014, 43, 4494.
[7] Hong, Y. N.; Tong, H.; Dong, Y. Q.; Haeussler, M.; Lam, J. W. Y.; Tang, B. Z. Abstr. Pap. Am. Chem. S. 2006, 232.
[8] (a) Zhou, J.; Chang, Z. F.; Jiang, Y. B.; He, B. R.; Du, M.; Lu, P.; Hong, Y. N.; Kwok, H. S.; Qin, A. J.; Qiu, H. Y.; Zhao, Z. J.; Tang, B. Z. Chem. Commun. 2013, 49, 2491;
(b) Zhang, J. Y.; Yang, Q. T.; Zhu, Y. X.; Liu, H. L.; Chi, Z. G.; Su, C. Y. Dalton. Trans. 2014, 43, 15785;
(c) Wu, J.; Sun, S.; Feng, X. Q.; Shi, J. B.; Hu, X. Y.; Wang, L. Y. Chem. Commun. 2014, 50, 9122.
[9] (a) Huang, Y. Y.; Hu, F.; Zhao, R.; Zhang, G. X.; Yang, H.; Zhang, D. Q. Chem. Eur. J. 2014, 20, 158;
(b) Zhang, X. Q.; Liu, M. Y.; Yang, B.; Zhang, X. Y.; Chi, Z. G.; Liu, S. W.; Xu, J. R.; Wei, Y. Polym. Chem. 2013, 4, 5060.
[10] (a) Tong, H.; Hong, Y. N.; Dong, Y. Q.; Haeussler, M.; Li, Z.; Lam, J. W. Y.; Dong, Y. P.; Sung, H. H. Y.; Williams, I. D.; Tang, B. Z. J. Phys. Chem. B 2007, 111, 11817;
(b) Shustova, N. B.; Cozzolino, A. F.; Reineke, S.; Baldo, M.; Dinca, M. J. Am. Chem. Soc. 2013, 135, 13326;
(c) Dong, W. Y.; Fei, T.; Palma-Cando, A.; Scherf, U. Polym. Chem. 2014, 5, 4048;
(d) Zhao, G. S.; Shi, C. X.; Guo, Z. Q.; Zhu, W. H.; Zhu, S. Q. Chin. J. Org. Chem. 2012, 32, 1620. (赵国生, 史川兴, 郭志前, 朱为宏, 朱世琴, 有机化学, 2012, 32, 1620.)
[11] (a) Shih, P. I.; Chuang, C. Y.; Chien, C. H.; Diau, E. W. G.; Shu, C. F. Adv. Funct. Mater. 2007, 17, 3141;
(b) Luo, J. F.; Wang, X. H.; Wang, X. M.; Su, W. M.; Tao, X. T.; Chen, Z. G. Chin. J. Chem. 2012, 30, 2488.
[12] (a) Gust, D. J. Am. Chem. Soc. 1977, 99, 6980;
(b) Keegstra, M. A.; DeFeyter, S.; DeSchryver, F. C.; Mullen, K. Angew. Chem., Int. Ed. 1996, 35, 774.
[13] Chen, J. P.; Han, Y. B.; Li, Y. Imaging Science and Photochemistry 2014, 32, 484. (陈金平, 韩永滨, 李嫕, 影像科学与光化学, 2014, 32, 484.)
[14] (a) Hu, R. R.; Lam, J. W. Y.; Liu, Y.; Zhang, X.; Tang, B. Z. Chem. Eur. J. 2013, 19, 5617;
(b) Li, P.; Zeng, Y.; Chen, J. P.; Li, Y. Y.; Li, Y. Acta Chim. Sinica 2012, 70, 1611. (李鹏, 曾毅, 陈金平, 李迎迎, 李嫕, 化学学报, 2012, 70, 1611.)
[15] (a) Dong, Y. Q.; Lam, J. W. Y.; Qin, A. J.; Liu, J. Z.; Li, Z.; Tang, B. Z. Appl. Phys. Lett. 2007, 91, 011111;
(b) Tang, H. Y.; Zeng, Y.; Li, Y. Y.; Chen, J. P.; Li, Y. Acta Chim. Sinica 2011, 69, 2241. (唐海云, 曾毅, 李迎迎, 陈金平, 李嫕, 化学学报, 2011, 69, 2241.)
/
| 〈 |
|
〉 |