

Spectral Properties of Water-Soluble Conjugated Polymer PFP/DNA/Porphyrin Complex
Received date: 2015-03-22
Online published: 2015-05-13
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21333012, 21373232) and the Chinese Academy of Sciences (No. XDB12020200).
Photodynamic therapy (PDT) is a particular treatment of tumors, which localizes the biological responses by the way that light is applied. In this paper, we have designed a water-soluble system potentially for PDT, which could selectively generate singlet oxygen efficiently under both of one- and two-photon excitation. This complex consists of a cationic water-soluble conjugated polymer PFP, anionic DNA and a cationic porphyrin. The cationic conjugated polymer PFP and porphyrin are connected by anionic DNA through electrostatic interactions. Energy transfer from conjugated polymer PFP (donor) to porphyrin (acceptor) is available since the considerable overlapping between the emission of donor and the absorption of acceptor (S0→S2). Steady-state spectra and fluorescence lifetime measurements have been performed on the complex with different DNA sequences in order to achieve the energy transfer efficiency. The results of steady-state fluorescence spectra show that there is a remarkable change of fluorescence intensity of the complex with G-quadruplex DNA, which indicates significant energy transfer from the donor to the acceptor. Furthermore, the greatest change of fluorescence lifetime measured by means of time-correlated single photon counting (TCSPC) also proves the highest energy transfer efficiency of the system with G-quadruplex DNA. Singlet oxygen quantum yield can be obtained by the detection of singlet oxygen emission spectra. Porphyrin bound to G-quadruplex DNA has the highest singlet oxygen quantum yield, which suggests this system a possible application in PDT since singlet oxygen could kill tumor cells. Two-photon absorption cross section has also been measured, which reflects the light-harvesting efficiency of the conjugated polymer PFP. The result shows that the conjugated polymer PFP owns large two-photon absorption cross section in the region of 720~850 nm where the transmission in tissue is higher compared to the visible light. The energy transfer efficiency and singlet oxygen quantum yield are both selective for different DNA sequences. G-quadruplex DNA that is very rich in tumor cells has the highest selectivity, which makes this system a potential application in PDT.
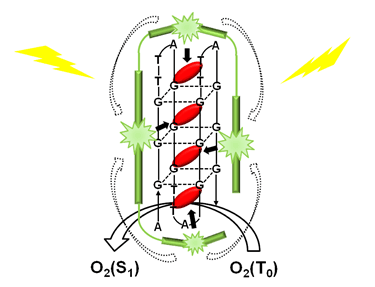
Key words: G-Quadruplex DNA; porphyrin; two-photon absorption; singlet oxygen; energy transfer
Long Saran , Wan Yan , Xia Andong . Spectral Properties of Water-Soluble Conjugated Polymer PFP/DNA/Porphyrin Complex[J]. Acta Chimica Sinica, 2015 , 73(7) : 723 -728 . DOI: 10.6023/A15030197
[1] Henderson, B. W.; Dougherty, T. J. Photochem. Photobiol. 1992, 55, 145.
[2] Dougherty, T. J.; Gomer, C. J.; Henderson, B. W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. J. Natl. Cancer Inst. 1998, 90, 889.
[3] Allen, C. M.; Sharman, W. M.; Van Lier, J. E. J. Porphyrins Phthalocyanines 2001, 5, 161.
[4] Jang, W.-D.; Nishiyama, N.; Zhang, G.-D.; Harada, A.; Jiang, D.-L.; Kawauchi, S.; Morimoto, Y.; Kikuchi, M.; Koyama, H.; Aida, T.; Kataoka, K. Angew. Chem., Int. Ed. 2005, 44, 419.
[5] Konan, Y. N.; Gurny, R.; Allémann, E. J. Photochem. Photobiol., B 2002, 66, 89.
[6] Haag, R.; Kratz, F. Angew. Chem., Int. Ed. 2006, 45, 1198.
[7] Boyle, R. W.; Dolphin, D. Photochem. Photobiol. 1996, 64, 469.
[8] Bonnett, R. Chem. Soc. Rev. 1995, 24, 19.
[9] Bonnett, R.; Martínez, G. Tetrahedron 2001, 57, 9513.
[10] DeRosa, M. C.; Crutchley, R. J. Coord. Chem. Rev. 2002, 233–234, 351.
[11] Liu, J.; Zhao, Y. W.; Zhao, J. Q.; Xia, A. D.; Jiang, L. J.; Wu, S.; Ma, L.; Dong, Y. Q.; Gu, Y. H. J. Photochem. Photobiol., B 2002, 68, 156.
[12] An, J.-Y.; Hu, Y.-Z.; Jiang, L.-J. J. Photochem. Photobiol., B 1996, 33, 261.
[13] Huang, Q.; Deng, P.; Li, Z.; Zhou, H.; Hu, X.; Pan, Z. Acta Chim. Sinica 2010, 68, 1070. (黄齐茂, 邓鹏星, 李志远, 周红, 胡学雷, 潘志权, 化学学报, 2010, 68, 1070.)
[14] Wan, Y.; Jia, K.; Li, B.; Bo, Z.; Xia, A. Sci. China Chem. 2009, 52, 56.
[15] Cheong, W.-F.; Prahl, S. A.; Welch, A. J. IEEE J. Quantum Electron. 1990, 26, 2166.
[16] Stork, M.; Gaylord, B. S.; Heeger, A. J.; Bazan, G. C. Adv. Mater. 2002, 14, 361.
[17] Wang, S.; Liu, B.; Gaylord, B. S.; Bazan, G. C. Adv. Funct. Mater. 2003, 13, 463.
[18] Wang, S.; Gaylord, B. S.; Bazan, G. C. J. Am. Chem. Soc. 2004, 126, 5446.
[19] He, F.; Tang, Y.; Wang, S.; Li, Y.; Zhu, D. J. Am. Chem. Soc. 2005, 127, 12343.
[20] Xing, C.; Xu, Q.; Tang, H.; Liu, L.; Wang, S. J. Am. Chem. Soc. 2009, 131, 13117.
[21] Bao, B.; Ma, M.; Fan, Q.; Wang, L.; Huang, W. Acta Chim. Sinica 2013, 71, 1379. (鲍碧清, 马明风, 范曲立, 汪联辉, 黄维, 化学学报, 2013, 71, 1379.)
[22] Liu, X.; Cai, X.; Huang, Y.; Shi, L.; Fan, Q.; Huang, W. Acta Chim. Sinica 2014, 72, 440. (刘兴奋, 蔡小慧, 黄艳琴, 石琳, 范曲立, 黄维, 化学学报, 2014, 72, 440.)
[23] He, G. S.; Tan, L.-S.; Zheng, Q.; Prasad, P. N. Chem. Rev. 2008, 108, 1245.
[24] Kim, H. M.; Cho, B. R. Chem. Commun. 2009, 153.
[25] Wei, C.; Jia, G.; Yuan, J.; Feng, Z.; Li, C. Biochemistry 2006, 45, 6681.
[26] Neidle, S.; Parkinson, G. N. Curr. Opin. Struct. Biol. 2003, 13, 275.
[27] Keniry, M. A. Biopolymers 2000, 56, 123.
[28] Lv, W.; Li, N.; Li, Y.; Li, Y.; Xia, A. J. Am. Chem. Soc. 2006, 128, 10281.
[29] Lipscomb, L. A.; Zhou, F. X.; Presnell, S. R.; Woo, R. J.; Peek, M. E.; Plaskon, R. R.; Williams, L. D. Biochemistry 1996, 35, 2818.
[30] Guliaev, A. B.; Leontis, N. B. Biochemistry 1999, 38, 15425.
[31] Han, F. X.; Wheelhouse, R. T.; Hurley, L. H. J. Am. Chem. Soc. 1999, 121, 3561.
[32] Vialas, C.; Pratviel, G.; Meunier, B. Biochemistry 2000, 39, 9514.
[33] Siddiqui-Jain, A.; Grand, C. L.; Bearss, D. J.; Hurley, L. H. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 11593.
[34] Seenisamy, J.; Rezler, E. M.; Powell, T. J.; Tye, D.; Gokhale, V.; Joshi, C. S.; Siddiqui-Jain, A.; Hurley, L. H. J. Am. Chem. Soc. 2004, 126, 8702.
[35] Dixon, I. M.; Lopez, F.; Estève, J.-P.; Tejera, A. M.; Blasco, M. A.; Pratviel, G.; Meunier, B. ChemBioChem 2005, 6, 123.
[36] Seenisamy, J.; Bashyam, S.; Gokhale, V.; Vankayalapati, H.; Sun, D.; Siddiqui-Jain, A.; Streiner, N.; Shin-ya, K.; White, E.; Wilson, W. D.; Hurley, L. H. J. Am. Chem. Soc. 2005, 127, 2944.
[37] Dixon, I. M.; Lopez, F.; Tejera, A. M.; Estève, J.-P.; Blasco, M. A.; Pratviel, G.; Meunier, B. J. Am. Chem. Soc. 2007, 129, 1502.
[38] Joachimi, A.; Mayer, G.; Hartig, J. S. J. Am. Chem. Soc. 2007, 129, 3036.
[39] Zhang, X.; Chen, C. Acta Chim. Sinica 2012, 70, 2475. (张现侠, 陈灿玉, 化学学报, 2012, 70, 2475.)
[40] Pasternack, R. F.; Gibbs, E. J.; Villafranca, J. J. Biochemistry 1983, 22, 2406.
[41] Pasternack, R. F.; Gibbs, E. J.; Villafranca, J. J. Biochemistry 1983, 22, 5409.
[42] Anderson, M. n. E.; Barrett, A. G. M.; Hoffman, B. M. J. Inorg. Biochem. 2000, 80, 257.
[43] Tan, C.; Atas, E.; Müller, J. G.; Pinto, M. R.; Kleiman, V. D.; Schanze, K. S. J. Am. Chem. Soc. 2004, 126, 13685.
[44] Frederiksen, P. K.; McIlroy, S. P.; Nielsen, C. B.; Nikolajsen, L.; Skovsen, E.; Jørgensen, M.; Mikkelsen, K. V.; Ogilby, P. R. J. Am. Chem. Soc. 2005, 127, 255.
[45] Briñas, R. P.; Troxler, T.; Hochstrasser, R. M.; Vinogradov, S. A. J. Am. Chem. Soc. 2005, 127, 11851.
[46] Chen, C.-Y.; Tian, Y.; Cheng, Y.-J.; Young, A. C.; Ka, J.-W.; Jen, A. K. Y. J. Am. Chem. Soc. 2007, 129, 7220.
[47] Crosby, G. A.; Demas, J. N. J. Phys. Chem. 1971, 75, 991.
[48] Chen, F.; Zhang, W.; Jia, M.; Wei, L.; Fan, X.-F.; Kuo, J.-L.; Chen, Y.; Chan-Park, M. B.; Xia, A.; Li, L.-J. J. Phys. Chem. C 2009, 113, 14946.
[49] Bonnett, R.; McGarvey, D. J.; Harriman, A.; Land, E. J.; Truscott, T. G.; Winfield, U. J. Photochem. Photobiol. 1988, 48, 271.
[50] Drobizhev, M.; Stepanenko, Y.; Dzenis, Y.; Karotki, A.; Rebane, A.; Taylor, P. N.; Anderson, H. L. J. Am. Chem. Soc. 2004, 126, 15352.
[51] Xu, C.; Webb, W. W. J. Opt. Soc. Am. B 1996, 13, 481.
[52] Rumi, M.; Ehrlich, J. E.; Heikal, A. A.; Perry, J. W.; Barlow, S.; Hu, Z.; McCord-Maughon, D.; Parker, T. C.; Röckel, H.; Thayumanavan, S.; Marder, S. R.; Beljonne, D.; Brédas, J.-L. J. Am. Chem. Soc. 2000, 122, 9500.
/
| 〈 |
|
〉 |