

Study on the Synthesis of Novel Sugar Amino Acids
Received date: 2015-03-25
Online published: 2015-05-25
Supported by
Supporting information for this article is available free of charge via the Internet at http://sioc-journal.cn.Project supported by the National Science Foundation for Young Scientists of China (No. 21302068), the Natural Science Foundation of Jiangsu Province, China (No. BK20130127), the specialized Research Fund for the Doctoral Program of Higher Education (No. 20120093120002) and the Fundamental Research Funds for the Central Universities (No. JUSRP51319B, JUSRP51411B).
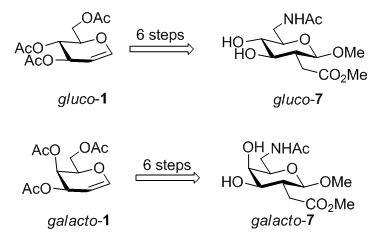
Sugar amino acids (SAAs) are carbohydrate derivatives bearing both amino and carboxylic acid functional groups. SAAs represent an important class of multifunctional building blocks, which are amenable to serve as glycomimetics or peptidomimetics with well-defined structures and useful properties. Because SAAs exist in nature in many forms with various biological activities, recently, many unnatural SAAs, as the demand for finding new molecules to discover new drugs and new materials, have been designed and synthesized by a number of research groups. In this paper, we have developed a convenient method for the synthesis of novel SAAs gluco-7 and galacto-7 for the first time. The structure of gluco-7 was similar to the natural SAA glucosaminuronic acid that was a component of many typical bacterial cell walls and could be used for the preparation of type D flu vaccine; while galacto-7 was similar to the natural SAA galactosaminuronic acid that was one of bacterial Vi-antigen components of Escherichia coli. Starting from unexpensive and commercially available 3,4,6-tri-O-acetyl-D-glucal and 3,4,6-tri-O-acetyl-D-galactal, two novel SAAs gluco-7 and galacto-7 were achieved in the linear 6 steps with 34% overall yield and 19% overall yield, respectively. The key reactions included radical addition, decarboxylation, iodine generation reaction, azide reaction and reductive amination reaction. The crucial step was the synthesis of the target compound gluco-7 from gluco-6. By using method A, the target compound gluco-7 was obtained in 4 steps with 63% overall yield. To optimize the transformation from gluco-6 to gluco-7, method B was developed to generate gluco-7 by using one-pot reaction successfully with 76% yield only in one step. It proved that method B was superior to method A with shorter steps and higher yields. All the new compounds were characterized by IR, 1H NMR, 13C NMR and HRMS data. Study on the synthesis and biological evaluation of linear and cyclic oligomers derived from gluco-7 and galacto-7 are currently in progress.
Key words: sugar amino acids; glycal; radical addition; one-pot reaction; synthesis
Wang Xuebin , Wang Xiaoli , Hu Jing , Wang Zhaoya , Pimpalpalle Tukaram M , Linker Torsten , Yin Jian . Study on the Synthesis of Novel Sugar Amino Acids[J]. Acta Chimica Sinica, 2015 , 73(7) : 699 -704 . DOI: 10.6023/A15030205
[1] Dondoni, A.; Marra, A. Chem. Rev. 2000, 100, 4395.
[2] Chakraborty, T. K.; Srinivasu, P.; Tapadar, S.; Mohan, B. K. J. Chem. Sci. 2004, 116, 187.
[3] (a) Chen, X.; Varki, A. ACS Chem. Biol. 2010, 5, 163;
(b) Wang, R.-Y.; Zhang, S.; Tang, Y.-H.; Hong, W.-Y.; Cheng, B.; Chen, Q.-X.; Zhu, W.-T.; Feng, L.-S. Chinese J. Org. Chem. 2014, 34, 461. (王汝一, 张姝, 谭艳红, 洪伟耀, 成波, 陈庆鑫, 朱蕴韬, 冯连顺, 有机化学, 2014, 34, 461.)
[4] Knapp, S.; Zhao, D. Org. Lett. 2000, 2, 4037.
[5] Gruner, S. A.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491.
[6] Heyns, K.; Kiessling, G.; Lindenberg, W.; Paulsen, H.; Webster, M. E. Chem. Ber. 1959, 92, 2435.
[7] Heyns, K.; Paulsen, H. Chem. Ber. 1955, 88, 188.
[8] Graf von Roedern, E.; Kessler, H. Angew. Chem. Int. Ed. Engl. 1994, 33, 687.
[9] Recent review:
(a) Risseeuw, M.; Overhand, M.; Fleet, G. W.; Simone, M. I. Amino Acids 2013, 45, 613;
(b) Gruner, S. A.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491; Selected papers:
(c) Chen, L.; Liang, F.-F.; Xu, M. F.; Xing, G.-W.; Deng, Z.-W. Acta Chim. Sinica 2009, 67, 1355. (陈力, 梁芬芬, 许美凤, 邢国文, 邓志威, 化学学报, 2009, 67, 1355);
(d) Simone, M. I.; Edwards, A. A.; Tranter, G. E.; Fleet, G. W. Amino Acids 2011, 41, 643;
(e) Knijnenburg, A. D.; Tuin, A. W.; Spalburg, E.; de Neeling, A. J.; Mars-Groenendijk, R. H.; Noort, D.; Otero, J. M.; Llamas-Saiz, A. L.; van Raaij, M. J.; van der Marel G. A.; Overkleeft, H. S.; Overhand, M. Chem. Eur. J. 2011, 17, 3995;
(f) Siriwardena, A.; Pulukuri, K. K.; Kandiyal, P. S.; Roy, S.; Bande, O.; Ghosh, S.; Garcia Fernández, J. M.; Martin, F. A.; Ghigo, J. M.; Beloin, C.; Ito, K.; Woods, R. J.; Ampapathi, R. S.; Chakraborty, T. K. Angew. Chem. Int. Ed. Engl. 2013, 52, 10221;
(g) Feher-Voelger, A.; Borges-González, J.; Carrillo, R. Chem. Eur. J. 2014, 20, 4007;
(h) Gajendra, S.; Uttam, G.; Sudip, P.; Ravi, S. A.; Tushar, K. C. Tetrahedron 2014, 70, 7681.
[10] (a) Linker, T.; Hartmann, K.; Sommermann, T.; Scheutzow, D.; Ruckdeschel, E. Angew. Chem. Int. Ed. Engl. 1996, 35, 1730;
(b) Linker, T.; Sommermann, T.; Kahlenberg, F. J. Am. Chem. Soc. 1997, 119, 9337;
(c) Sommermann, T.; Kim, B. G.; Peters, K.; Peters, E. M.; Linker, T. Chem. Commun (Camb). 2004, 22, 2624;
(d) Linker, T.; Schanzenbach, D.; Elamparuthi, E.; Sommermann, T.; Fudickar, W.; Gyóllai, V.; Somsák, L.; Demuth, W.; Schmittel, M. J. Am. Chem. Soc. 2008, 130, 16003;
(e) Elamparuthi, E.; Linker, T. Org. Lett. 2008, 10, 1361;
(f) Elamparuthi, E.; Linker, T. Angew. Chem. Int. Ed. Engl. 2009, 48, 1853.
[11] (a) Yin, J.; Spindler, J.; Linker, T. Chem. Commun. (Camb). 2007, 26, 2712;
(b) Yin, J.; Sommermann, T.; Linker, T. Chem. Eur. J. 2007, 13, 10152;
(c) Yin, J.; Linker, T. Org. Biomol. Chem. 2012, 10, 2351.
/
| 〈 |
|
〉 |