

Effects of Surface Modification of Carbon Nanofibers on Their Electro- catalytic Activity for Hydrogen Evolution Reaction of Water Electrolysis
Received date: 2015-03-21
Online published: 2015-06-29
Supported by
Supporting information for this article is available free of charge via the Internet at http://sioc-journal.cn.Project supported by the National Natural Science Foundation of China (No. 21073061) and Shanghai university student innovative activities plan.
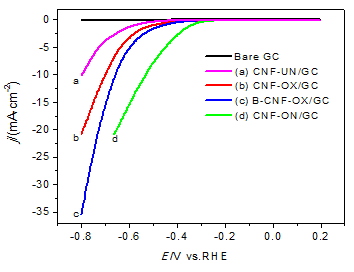
The effects of surface modification of CNF on their electrocatalytic activity for hydrogen evolution reaction (HER) were investigated. Firstly, Oxygen-containing functional groups were introduced onto the CNF surface (labeled as CNF-OX) by a simple sonochemical oxidation in mixed acids (concentrated sulfuric acid and nitric acid) and then the nitrogen-containing functional groups were introduced onto the CNF-OX surface (labeled as CNF-ON) by sonochemical treatment in ammonia, and the B-doped CNF was synthesized by mixing CNF-OX and boric acid in a ratio following by pyrolysis at 800 ℃ under N2 atmosphere (labeled as B-CNF-OX). The XPS results showed that the ultrasonic treatments could introduce oxygen- and nitrogen-containing functional groups onto the CNF surface successfully, the oxygen atoms content of CNF-OX was increased from 1.96% to 6.01% compared to the untreated CNF (CNF-UN), and nitrogen atoms content of CNF-ON was increased from 0% to 1.02% compared to CNF-OX. The pyrolysis of boric acid could introduce B element onto the CNF-OX surface where the B atoms content of B-CNF-OX was 1.00%. The linear sweep voltammetry (LSV) test results showed that the HER electrocatalytic activity of the modified CNFs were higher than CNF-UN, and among them CNF-ON had the highest electrocatalytic activities for HER than the others, the onset overpotential of CNF-ON was 344 mV, which shifted 240 mV positively compared to CNF-UN, and the electrocatalytic activity of B-CNF-OX was enhanced by B-doping treatment compared to CNF-OX. The Tafel test indicated CNF-ON had smallest Tafel slop of 154 mV/dec and largest exchange current density of 6.68 μA/cm2. The electrochemical impedance spectroscopy (EIS) was carried out to investigate the complex interfacial properties of the CNFs modified electrode, and the results showed that CNF-ON had the smallest charge transfer resistance of 1578 Ω among all the catalysts, which means the nitrogen-doped CNF had higher activity than the oxygen- and boron-doped CNF. All above indicated that the doping of heteroatom (O, B, N) onto the CNFs surface had effects on their electrocatalytic activity for HER, especially the introduction of N atom onto the CNF surface had excellent improvement.
Niu Dongfang , Ding Yong , Ma Zhixing , Wang Minghui , Liu Zhou , Zhang Bowen , Zhang Xinsheng . Effects of Surface Modification of Carbon Nanofibers on Their Electro- catalytic Activity for Hydrogen Evolution Reaction of Water Electrolysis[J]. Acta Chimica Sinica, 2015 , 73(7) : 729 -734 . DOI: 10.6023/A15030195
[1] Liu, L.; Zha, D.-W.; Wang, Y.; He, J.-B. Int. J. Hydrogen Energy 2014, 39, 14712.
[2] Cao, X.; Han, Y.; Gao, C.; Xu, Y.; Huang, X.; Willander, M.; Wang, N. Nano Energy 2014, 9, 301.
[3] Bessel, C. A.; Laubernds, K.; Rodriguez, N. M.; Baker, R. T. K. J. Phys. Chem. B 2001, 105, 1115.
[4] Aricò, A. S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Van Schalkwijk, W. Nat. Mater. 2005, 4, 366.
[5] Taylor, R.; Humffray, A. J. Electroanal. Chem. Interfacial Electrochem. 1975, 64, 63.
[6] Zhong, R.-S.; Qin, Y.-H.; Niu, D.-F.; Zhang, X.-S.; Zhou, X.-G.; Sun, S.-G.; Yuan, W.-K. Electrochim. Acta 2013, 89, 157.
[7] Qin, Y.-H.; Jiang, Y.; Niu, D.-F.; Zhang, X.-S.; Zhou, X.-G.; Niu, L.; Yuan, W.-K. J. Power Sources 2012, 215, 130.
[8] Qin, Y.-H.; Li, H.-C.; Yang, H.-H.; Zhang, X.-S.; Zhou, X.-G.; Niu, L.; Yuan, W.-K. J. Power Sources 2011, 196, 159.
[9] Zhang, Z.-G.; Zhao, T.-K.; Li, T.-H.; Wang, J.-B.; Guo, Z.-X. Carbon 2014, 26. (张智广, 赵廷凯, 李铁虎, 王建兵, 郭争光, 炭素, 2014, 26.)
[10] Cao, Y.; Yu, H.; Tan, J.; Peng, F.; Wang, H.; Li, J.; Zheng, W.; Wong, N.-B. Carbon 2013, 57, 433.
[11] Zhao, D.-M.; Li, Z.-W.; Liu, L.-D.; Zhang, Y.-H.; Ren, D.-C.; Li, J. Acta Chim. Sinica 2013, 72, 185. (赵冬梅, 李振伟, 刘领弟, 张艳红, 任德财, 李坚, 化学学报, 2013, 72, 185.)
[12] Lu, Z.-J.; Xu, M.-W.; Bao, S.-J.; Chai, H. Acta Chim. Sinica 2013, 71, 957. (鲁振江, 徐茂文, 包淑娟, 柴卉, 化学学报, 2013, 71, 957.)
[13] Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. ACS nano 2010, 4, 1321.
[14] Li, Y.; Mi, R.; Li, S.; Liu, X.; Ren, W.; Liu, H.; Mei, J.; Lau, W.-M. Int. J. Hydrogen Energy 2014, 39, 16073.
[15] Cheng, Y.; Tian, Y.; Fan, X.; Liu, J.; Yan, C. Electrochim. Acta 2014, 143, 291.
[16] Li, L.-X.; Zhao, H.-W.; Xu, W.-W.; Zhang, Y.-Q.; An, B.-G.; Geng, X. Acta Phys.-Chim. Sin. 2015, 31(3), 498. (李莉香, 赵宏伟, 许微微, 张砚秋, 安百钢, 耿新, 物理化学学报, 2015, 31(3), 498.)
[17] Zhang, J.; Wu, S.; Chen, X.; Pan, M.; Mu, S. J. Power Sources 2014, 271, 522.
[18] Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Carbon 2014, 73, 361.
[19] Zhong, R.-S.; Qin, Y.-H.; Niu, D.-F.; Tian, J.-W.; Zhang, X.-S.; Zhou, X.-G.; Sun, S.-G.; Yuan, W.-K. J. Power Sources 2013, 225, 192.
[20] Moncoffre, N.; Hollinger, G.; Jaffrezic, H.; Marest, G.; Tousset, J. Nucl. Instrum. Methods Phys. Res., Sect. B 1985, 7, 177.
[21] Figueiredo, J. L.; Pereira, M. F. R. Catal. Today 2010, 150, 2.
[22] Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. J. Am. Chem. Soc. 2013, 135, 17881.
[23] Liu, B.; He, J.-B.; Chen, Y.-J.; Wang, Y.; Deng, N. Int. J. Hydrogen Energy 2013, 38, 3130.
[24] Jiang, Y.; Zhang, J.; Qin, Y.-H.; Niu, D.-F.; Zhang, X.-S.; Niu, L.; Zhou, X.-G.; Lu, T.-H.; Yuan, W.-K. J. Power Sources 2011, 196, 9356.
/
| 〈 |
|
〉 |