

Effects of Co/SiO2 Particle Size on Fischer-Tropsch Synthesis: Study by TPD and DRIFTS
Received date: 2015-02-15
Online published: 2015-07-07
Supported by
Supporting information for this article is available free of charge via the Internet at http://sioc-journal.cn.Project supported by the National Natural Science Foundation of China (No. 21273261).
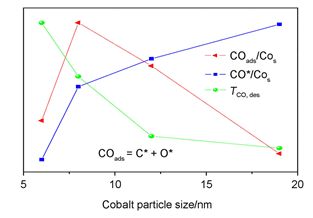
The effects of cobalt particle size are still controversial for whether it influences Fischer-Tropsch synthesis (F-T synthesis) behavior intrinsically. In the F-T synthesis, a large number of different reaction pathways as well as multitude of products does result in many difficulties in the search for the intrinsic causes. The adsorption and dissociation of CO and H2 is a key step for Fischer-Tropsch synthesis. So the effects are trying to be explained by means of exploring the relationship between the adsorbed behavior of CO and H2 and cobalt particle size. In this work, four Co/SiO2 catalysts with different cobalt particle sizes, named 6, 8, 12, 19 nm, were prepared by incipient wetness impregnation using ethylene glycol (EG) and water mixture as a solvent. The catalysts were characterized by N2 physisorption, X-Ray powder diffraction (XRD), transmission electron microscopy (TEM), temperature programmed desorption (TPD), diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and temperature programmed surface reaction (TPSR), and their Fischer-Tropsch reactivity were tested in a micro-fixed bed reactor. The F-T synthesis test showed that the catalysts with larger cobalt particle size had lower CO conversion, but their apparent Turnover Frequency (TOF) displayed a maximum for the catalyst with cobalt particle size of 8 nm. TPD and DRIFTS results indicated that both the adsorption and dissociation of CO were stronger on smaller cobalt particle, while some of the active sites could be blocked by carbon species on the surface, thus decreased the effective active sites. The adsorption of CO were weaker on larger cobalt particle and the formed C* species were easily desorbed, exhibiting higher CO*/Cos ratio on the surface. Therefore lower activity and higher CO2 selectivity were observed. It is suggested that the catalyst with medium cobalt particle size can produce medium adsorbability of CO and proper amount of COads (surface adsorbed CO) and C* species to balance suitable C/H ratio on the surface, exhibiting higher F-T activity and selectivity.
Key words: particle size; cobalt-based catalysts; F-T synthesis; TPD; DRIFTS
Qiu Chengwu , Wu Baoshan , Meng Shaocong , Li Yongwang . Effects of Co/SiO2 Particle Size on Fischer-Tropsch Synthesis: Study by TPD and DRIFTS[J]. Acta Chimica Sinica, 2015 , 73(7) : 690 -698 . DOI: 10.6023/A15020133
[1] Song, D.; Li, J.; Cai, Q. J. Phys. Chem. C 2007, 111, 18970.
[2] Sun, Y. H.; Chen, J. G.; Wang, J. G.; Jia, L. T.; Hou, B.; Li, D. B.; Zhang, J. J. Chin. Catal. 2010, 31, 919. (孙予罕, 陈建刚, 王俊刚, 贾丽涛, 侯博, 李德宝, 张娟, 催化学报, 2010, 31, 919.)
[3] Botes, F. G.; Niemantswerdriet, J. W.; Van de Loosdrecht, J. Catal. Today 2013, 215, 112.
[4] Wang, Z. J.; Skiles, S.; Yang, F.; Yan, Z.; Goodman, D. W. Catal. Today 2012, 181, 75.
[5] Azzam, K.; Jacobs, G.; Ma, W. Catal. Lett. 2014, 144, 389.
[6] Van Steen, E.; Claeys, M. Chem. Eng. Technol. 2008, 31(5), 655.
[7] Den Breejen, J. P.; Radstake, P. B.; Bezemer, G. L.; Bitter, J. H.; Frøseth, V.; Holmen, A.; De Jong K. P. J. Am. Chem. Soc. 2009, 131, 7197.
[8] Wood, D. M. Phys. Rev. Lett. 1981, 46(11), 749.
[9] Fischer, N.; Van Steen, E.; Claeys, M. J. Catal. 2013, 299, 67.
[10] Prieto, G.; Martínez, A.; Concepción, P.; Moreno-Tost, R. J. Catal. 2009, 266, 129.
[11] Smith, M. L.; Kumar, N.; Spivey, J. J. J. Phys. Chem. C 2012, 116, 7931.
[12] Gao, H. Y. Ph. D. Dissertation, Institute of Coal Chemistry, Chinese Academy of Science, Taiyuan, 2003. (高海燕, 博士论文, 中国科学院山西煤炭化学研究所, 太原, 2003. )
[13] Koizumia, N.; Suzukia, S.; Niiyama, S.; Ibi, Y.; Shindo, T.; Yamada, M. Appl. Catal. A: Gen. 2011, 395, 138.
[14] Borg, Ø.; Dietzel, P. D. C.; Spjelkavik, A. I.; Tveten, E. Z.; Walmsley, J. C.; Diplas, S.; Eri, S.; Holmen, A.; Rytter, E. J. Catal. 2008, 259, 161.
[15] Reuel, R. C.; Bartholomew, C. H. J. Catal. 1984, 85, 63.
[16] Ji, L.; Lin, J.; Zeng, H. C. J. Phys. Chem. B 2000, 104, 1783.
[17] Zhang, J. L.; Ren, J.; Chen, J. G.; Sun, Y. H. Acta Phys. Chim. Sin. 2002, 18(3), 260. (张俊岭, 任杰, 陈建刚, 孙予罕, 物理化学学报, 2002, 18(3), 260. )
[18] Du?, R.; Lisowski, W. Surf. Sci. 1976, 61, 635.
[19] Barbier, A.; Tuel, A.; Arcon, I.; Kodre, A.; Martin, G. A. J. Catal. 2001, 200, 106.
[20] Bartholomew, C. H. Catal. Lett. 1990, 7, 27.
[21] Rodrigues, E. L.; Bueno, J. M. C. Appl. Catal. A: Gen. 2004, 257, 201.
[22] Nowitzki, T.; Borchert, H.; Jurgens, B.; Risse, T.; Zielasek, V.; Baumer, M. ChemPhysChem 2008, 9, 729.
[23] Pauls, C.; Przyrembel, D.; Christmann, K. J. Phys. Chem. B 2004, 108, 14749.
[24] Galhenage, R. P.; Yan, H.; Ahsen, A. S.; Ozturk, O.; Chen, D. A. J. Phys. Chem. C 2014, 118(31), 17773.
[25] Henry, C. R.; Chapon, C.; Goyhenex, C.; Monot, R. Surf. Sci. 1992, 272(1-3), 283.
[26] Schweicher, J. Ph. D. Dissertation, Universite Librede Bruxelles, Bruxelles, 2010.
[27] Swart, I.; De Groot, F. M. F.; Weckhuysen, B. M.; Rayner, D. M.; Meijer, G.; Fielicke, A. J. Am. Chem. Soc. 2008, 130, 2126.
[28] Adachi, M.; Yoshii, K.; Han, Y. Z.; Fujimoto, K. Bull. Chem. Soc. Jpn. 1996, 69, 1509.
[29] Kumar, N.; Jothimurugesan, K.; Stanley, G. G.; Schwartz, V.; Spivey, J. J. J. Phys. Chem. C 2011, 115, 990.
[30] Bian, G.; Nanba, T.; Koizumi, N.; Yamada, M. J. Mol. Catal. A: Chem. 2002, 178, 219.
[31] Ciobîcã, I. M.; Van Santen, R. A.; Van Berge, P. J.; Van de Loosdrecht, J. Surf. Sci. 2008, 602, 17.
[32] Rane, S.; Borg, Ø.; Rytter, E.; Holmen, A. Appl. Catal. A: Gen. 2012, 437-438, 10.
[33] Park, J. Y.; Lee, Y. J.; Karandikar, P. R.; Jun, K. W.; Ha, K. S.; Park, H. G. Appl. Catal., A 2011, 411-412, 15.
[34] Song, D.; Li, J. J. Mol. Catal. A: Chem. 2006, 247, 206.
/
| 〈 |
|
〉 |