

Supramolecular Gels Formation from N,N',N"-Tris(4-pyridyl)trimesic Amide Induced by Water
Received date: 2015-05-10
Online published: 2015-07-23
Supported by
Project supported by the National Natural Science Foundation of China (No. 51268002), the Natural Science Foundation of Jiangxi Province (Nos. 20142BCB22007, 20143ACB20009), the Natural Science Foundation of Jiangxi Province (No. KJLD14080), and the “Gan Po 555 Engineering Excellence” of Jiangxi Province, China.
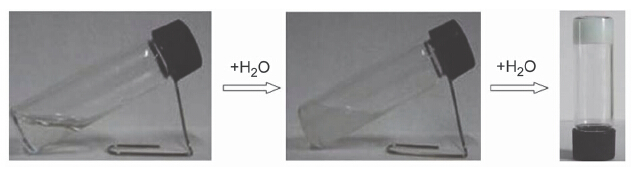
N,N',N"-Tris(4-pyridyl)trimesic amide (4-btapa) was synthesized by chemical modification using 4-aminopyridine to benezene-1,3,5-tricarboxylic acid. The gelating ability of 4-btapa to different solvents and the effect of pH on gelating ability were studied in detail. The results indicated that a stable gel was obtained by adding a certain amount of water to a 4-btapa DMSO solution at room temperature. While adding a certain amount of water to solutions of 4-btapa in DMF, THF, methanol and so on, respectively, only flow turbid liquids with large viscosity were obtained. Upon heating the obtained turbid liquids to clear solutions and cooling them at room temperature, stable gels were also obtained. The structures of the aggregates in the gels were investigated using Fourier-transform infrared spectroscopy (FT-IR), nuclear magnetic resonance (NMR) spectroscopy, small angle X-ray scattering (SAXS) and scanning electron microscopy (SEM). The result of SEM shows that the 4-btapa can form fibrous self-assembly aggregates in different aqueous solvent, while 4-btapa shows the fiber cluster structure at pH=3.0 water-ethanol system, which is different from regular fibrous aggregate in pH=7.0, suggesting that the pH can affect the aggregation morphology of gelators. The results of nuclear magnetic resonance and infrared spectroscopy revealed that the molecular hydrogen bonds of N—H…Py and aromatic π-π stacking interactions were formed in gel state and water was a part of the gel. From the above observation and analysis of FT-IR, 1H-NMR and XRD results, we can suggest the self-assembly arrangement of 4-btapa in gel state.
Luo Haiqing , Liu Qihao , Zhu Wancai , Wang Kejun , Luo Xuzhong . Supramolecular Gels Formation from N,N',N"-Tris(4-pyridyl)trimesic Amide Induced by Water[J]. Acta Chimica Sinica, 2015 , 73(10) : 1031 -1037 . DOI: 10.6023/A15050321
[1] Tokuyama, H.; Iwama, T. Langmuir 2007, 23, 13104.
[2] Estroff, L. A.; Hamilton, A. D. Chem. Rev. 2004, 4, 1201.
[3] Sun, S.; Liu, J.; Gao, D.; He, P. L.; Fang, Y. Acta Chim. Sinica 2010, 68, 1174. (孙松, 刘静, 高迪, 何盼丽, 房喻, 化学学报, 2010, 68, 1174.)
[4] Shao, Y.; Li, C.; Zhou, X.; Chen, P.; Yang, Z. Q.; Li, Z. B.; Liu, D. S. Acta Chim. Sinica 2015, 73, 815. (邵昱, 李闯, 周旭, 陈平, 杨忠强, 李志波, 刘冬生, 化学学报, 2015, 73, 815.)
[5] Terech, P.; Weiss, R. G. Chem. Rev. 1997, 97, 3133.
[6] Zhang, Y. L.; Yang, B.; Xu, L. X.; Zhang, X. Y.; Tao, L.; Wei, Y. Acta Chim. Sinica 2013, 71, 485. (张亚玲, 杨斌, 许亮鑫, 张小勇, 陶磊, 危岩, 化学学报, 2013, 71, 485.)
[7] Liu, S. L.; Zhou, Y. G.; Chen, F. H.; Zhu, S. J.; Su, F. Acta Chim. Sinica 2015, 73, 47. (刘水莲, 周洋, 陈福花, 朱寿进, 宿烽, 李速明, 化学学报, 2015, 73, 47.)
[8] Shen, L. Y.; Chen, X. Z.; Yu, H. T.; Liang, G. Chin. J. Org. Chem. 2009, 29, 321. (沈利英, 陈肖卓, 于海涛, 梁刚, 有机化学, 2009, 29, 321.)
[9] Haines, S. R.; Harrison, R. G. Chem. Commun. 2002, 2846.
[10] Li, Y. G.; Liu, K. Q.; Liu, J.; Peng, J. X.; Feng, X. L.; Fang, Y. Langmuir 2006, 22, 7016.
[11] Shome, A.; Debnath, S.; Das, P. K. Langmuir 2008, 24, 4280.
[12] Luo, X. Z.; Deng, J. H.; Zhong, J. L.; Liu, H. J.; Wang, K. J.; Zhong, D. C. J. Am. Chem. Soc. 2013, 135, 11684.
[13] Fu, X. J.; Yang, Y. J. J. Colloid Interface Sci. 2007, 315, 376.
[14] Huang, X. T.; Erech, P.; Raghavan, S. R.; Weiss, R. G. J. Am. Chem. Soc. 2005, 127, 4336.
[15] Luo, X. Z.; Wang, Q.; Zhong, J. L.; Pan, H.; Chen, Z. X. Acta Phys. Chim. Sin. 2011, 27, 1719. (罗序中, 王琼, 钟金莲, 潘虹, 陈志兴. 物理化学学报, 2011, 27, 1719.)
[16] Liang, Y. Q.; Jiang, Y. T.; Tian, Y. C. Acta Phys. Chim. Sin. 1991, 7, 72. (梁映秋, 姜玉涛, 田永驰, 物理化学学报, 1991, 7, 72.)
[17] Shi, N.; Dong, H.; Yin, G.; Xu, Z.; Li, S. H. Adv. Funct. Mater. 2007, 17, 1837.
[18] Jung, J. H.; Shinkai, S.; Shimizu, T. Chem. Eur. J. 2002, 8, 2684.
[19] Snip, E.; Shinkai, S.; Reinhoudt, D. N. Tetrahedron Lett. 2001, 42, 2153.
[20] Bhattacharya, S.; Basit, H. Chem. Eur. J. 2008, 14, 6534.
[21] Zhou, S. L.; Matsumoto, S.; Tian, H. D.; Yamane, H.; Ojida, A.; Kiyonaka, S.; Hamachi, I. Chem. Eur. J. 2005, 11, 1130.
[22] Kiyonaka, S.; Zhou, S. L.; Hamachi, I. Supramol. Chem. 2003, 15, 521.
[23] Kiyonaka, S.; Sugiyasu, K.; Shinkai, S.; Hamachi, I. J. Am. Chem. Soc. 2002, 124, 10954.
[24] de Loos, M.; Feringa, B. L. Tetrahedron 2007, 63, 7285.
[25] Hasegawa, S.; Kitagawa, S. J. Am. Chem. Soc. 2007, 129, 2607.
/
| 〈 |
|
〉 |