

Tandem Heck Reaction of Tertiary Enamides: A Novel Access to trans-2,5-Disubstituted-3-Pyrroline Derivatives
Received date: 2015-06-08
Online published: 2015-08-18
Supported by
Project supported by the National Natural Science Foundation of China (No. 21320102002).
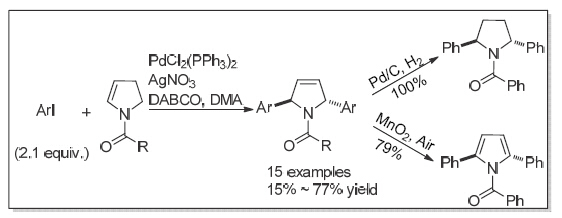
3-Pyrroline structure is a common scaffold in natural products and synthetic bioactive molecules. 3-Pyrroline derivatives are also important intermediates in organic synthesis. The synthetic study of 3-pyrroline compounds has therefore always attracted great attention. Tertiary enamides are a class of unique and versatile synthons. They are able to participate in intramolecular and intermolecular reactions with various electrophiles, affording diverse nitrogen-containing heterocyclic compounds. Recently, we attempted the Heck reaction of the five-membered cyclic tertiary enamides and discovered the formation of α-arylated product with the double bond being shifted. We then envisioned a tandem diarylation reaction to synthesize 2,5-disubstituted-3-pyrroline derivatives. Herein, we reported the investigation of the Heck reaction of cyclic tertiary enamides, a simple and convenient approach to trans-2,5-disubstituted-3-pyrrolines. Silver salts were found to be effective additives to accelerate the reaction, while PdCl2(PPh3)2 appeared as the best catalyst to improve the regioselectivity. Under the optimized conditions, a variety of differently substituted aryl iodides reacted smoothly with N-benzoyl-2,3- dihydro-1H-pyrrole 2a to afford the desired products 4a~4k in moderate to high yields. Other N-substituted enamide substrates 2b~2e underwent similar tandem reactions to give the corresponding products in moderate chemical yields. A representative procedure for this reaction is as follows: to an oven-dried Schlenk tube was successively added PdCl2(PPh3)2 (18 mg, 0.025 mmol, 5 mol%), AgNO3 (177 mg, 1.05 mmol, 2.1 equiv.), DABCO (168 mg, 1.5 mmol, 3.0 equiv.) and dry DMA (1.0 mL) under argon atmosphere. After stirring for 5 min at room temperature and the mixture turned black, aryl iodides 1a~1l (1.05 mmol) in DMA (1.0 mL) was introduced into the tube, following the addition of substrate 2a~2f (0.50 mmol) in DMA (1.0 mL). The resulting mixture was stirred at 80 ℃ until starting enamides and mono-arylated compounds were consumed, which was monitored by TLC analysis. After filtration, extraction and concentration in vacuo, the reaction residue was flash chromatographed on a silica gel column eluted with a mixture of petroleum ether and ethyl acetate (V:V=6:1) to give the pure product 4a~4p. The resulting trans-2,5-diphenyl-3-pyrroline was converted to pyrrole and pyrrolidine derivative, respectively, by oxidation and reduction.
Key words: tertiary enamide; Heck reaction; tandem reaction; 3-pyrroline
He Ling , Gu Mengdi , Wang Dexian , Zhao Liang , Wang Meixiang . Tandem Heck Reaction of Tertiary Enamides: A Novel Access to trans-2,5-Disubstituted-3-Pyrroline Derivatives[J]. Acta Chimica Sinica, 2015 , 73(10) : 1018 -1024 . DOI: 10.6023/A15060400
[1] For recent reviews of pyrroline, pyrrole, pyrrolidine containing natural products see: (a) O'Hagan, D. Nat. Prod. Rep. 2000, 17, 435.
(b) Mauger, A. B. J. Nat. Prod. 1996, 59, 1205.
(c) Haslam, E. Shikimic Acid Metabolism and Metabolites, Wiley, New York, 1993.
[2] Chan, G. W.; Francis, T.; Thureen, D. R.; Offen, P. H.; Pierce, N. J.; Westley, J. W.; Johnson, R. K. J. Org. Chem. 1993, 58, 2544.
[3] Hamasaki, A.; Zimpleman, J. M.; Hwang, I.; Boger, D. L. J. Am. Chem. Soc. 2005, 127, 10767.
[4] Cox, C. D.; Coleman, P. J.; Breslin, M. J.; Whitman, D. B.; Garbaccio, R. M.; Fraley, M. E.; Buser, C. A.; Walsh, E. S.; Hamilton, K.; Schaber, M. D.; Lobell, R. B.; Tao, W.; Davide, J. P.; Diehl, R. E.; Abrams, M. T.; South, V. J.; Huber, H. E.; Torrent, M.; Prueksaritanont, T.; Li, C.; Slaughter, D. E.; Mahan, E.; Fernandez-Metzler, C.; Yan, Y.; Kuo, L. C.; Kohl, N. E.; Hartman, G. D. J. Med. Chem. 2008, 51, 4239.
[5] Ozawa, M.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Ohsaki, A. Bioorg. Med. Chem. Lett. 2009, 19, 234.
[6] Examples of 3-pyrrolines in the synthesis of natural products or as analgous of pharmaceutically relevant compounds: (a) Huwe, C. M.; Blechert, S. Tetrahedron Lett. 1995, 36, 1621.
(b) Bondzi?, B. P.; Eilbracht, P. Org. Lett. 2008, 10, 3433.
(c) Sampath, M.; Beatrix Lee, P.-Y.; Loh, T.-P. Chem. Sci. 2011, 2, 1988.
(d) Mycock, D. K.; Glossop, P. A.; Lewis, W.; Hayes, C. J. Tetrahedron Lett. 2013, 54, 55.
(e) Huang, P.-Q.; Ou, W.; Ye, J.-L. Chin. J. Chem. 2015, 33, 655.
[7] For selected examples of synthesis of 2,5-disubstituted 3-pyrroline derivatives using [3+2] cycloaddition of imines and allenes: (a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535.
(b) Zhu, X.-F.; Henry, C. E.; Kwon, O. Tetrahedron 2005, 61, 6276.
(c) Zhao, G.-L.; Shi, M. J. Org. Chem. 2005, 70, 9975.
(d) Zheng, S.; Lu, X. Org. Lett. 2008, 10, 4481.
[8] For example of synthesis of 2,5-disubstituted 3-pyrroline derivatives using [3+2] cycloaddition of imines with alkynes: Meng, L.-G.; Cai, P.; Guo, Q.; Xue, S. J. Org. Chem. 2008, 73, 8491.
[9] For an example of synthesis of 2,5-disubstituted 3-pyrroline derivatives via ring closing metathesis of diallyl amines: Evans, P. A.; Robinson, J. E. Org. Lett. 1999, 1, 1929.
[10] For selected examples of synthesis of 2,5-disubstituted 3-pyrroline derivatives using cyclization of amino allenes: (a) Dieter, R. K.; Yu, H. Org. Lett. 2001, 3, 3855.
(b) Xu, T.; Mu, X.; Peng, H.; Liu, G. Angew. Chem., Int. Ed. 2011, 50, 8176.
(c) Ohno, H.; Toda, A.; Miwa, Y.; Taga, T.; Osawa, E.; Yamaoka, Y.; Fujii, N.; Ibuka, T. J. Org. Chem. 1999, 64, 2992.
(d) Ma, S.; Yu, F.; Gao, W. J. Org. Chem. 2003, 68, 5943.
(e) Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634.
(f) Ohno, H.; Kadoh, Y.; Fujii, N.; Tanaka, T. Org. Lett. 2006, 8, 947.
(g) Brioche, J.; Meyer, C.; Cossy, J. Org. Lett. 2013, 15, 1626.
[11] For selected examples of synthesis of 2,5-disubstituted 3-pyrroline derivatives via [3+2] cycloaddition of alkynes with aziridines: (a) Anderson, W. K.; Milowsky, A. S. J. Med. Chem. 1986, 29, 2241.
(b) Li, L.; Zhang, J. Org. Lett. 2011, 13, 5940.
[12] For reviews on reactivity of enamides see: (a) Carbery, D. R. Org. Biomol. Chem. 2008, 6, 3455.
(b) Gopalaiah, K.; Kagan, H. B. Chem. Rev. 2011, 111, 4599.
[13] For a recent review on reactivity of tertiary enamides see: Wang, M.-X. Chem. Commun. 2015, 51, 6039.
[14] Yang, L.; Zheng, Q.-Y.; Wang, D.-X.; Huang, Z.-T.; Wang, M.-X. Org. Lett. 2008, 10, 2461.
[15] Tong, S.; Wang, D.-X.; Zhao, L.; Zhu, J.; Wang, M.-X. Angew. Chem., Int. Ed. 2012, 51, 4417.
[16] (a) Yang, L.; Lei, C.-H.; Wang, D.-X.; Huang, Z.-T.; Wang, M.-X. Org. Lett. 2010, 12, 3918.
(b) Yang, L.; Wang, D.-X.; Huang, Z.-T.; Wang, M.-X. J. Am. Chem. Soc. 2009, 131, 10390.
[17] Tong, S.; Yang, X.; Wang, D.-X.; Zhao, L.; Zhu, J.; Wang, M.-X. Tetrahedron 2012, 68, 6492.
[18] (a) Lei, C.-H.; Wang, D.-X.; Zhao, L.; Zhu, J.; Wang, M.-X. J. Am. Chem. Soc. 2013, 135, 4708.
(b) Lei, C.-H.; Wang, D.-X.; Zhao, L.; Zhu, J.; Wang, M.-X. Chem. Eur. J. 2013, 19, 16981.
(c) Lei, C.-H.; Zhao, L.; Wang, D.-X.; Zhu, J.; Wang, M.-X. Org. Chem. Front. 2014, 1, 909.
[19] He, L.; Zhao, L.; Wang, D.-X.; Wang, M.-X. Org. Lett. 2014, 16, 5972.
[20] He, L.; Liu, H.-B.; Zhao, L.; Wang, D.-X.; Wang, M.-X. Tetrahedron 2015, 71, 523.
[21] For selected examples of applications of Heck reaction of tertiary enamides in total synthesis of natural products: (a) Kawagishi, F.; Toma, T.; Inui, T.; Yokoshima, S.; Fukuyama, T. J. Am. Chem. Soc. 2013, 135, 13684.
(b) Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 6552.
[22] For recent review of Heck reaction of tertiary enamides see: (a) Cartney, D. M.; Guiry, P. J. Chem. Soc. Rev. 2011, 40, 5122.
(b) Li, H.; Ding, C.; Xu, B.; Hou, X. Acta Chim. Sinica 2014, 72, 765. (李浩, 丁昌华, 许斌, 侯雪龙, 化学学报, 2014, 72, 765.)
(c) Shibasaki, M.; Vogl, E. M.; Ohshima, T. Adv. Synth. Catal. 2004, 346, 1533.
[23] For selected examples of Heck reaction of five-membered tertiary enamides: (a) Nilsson, K.; Hallberg, A. J. Org. Chem. 1990, 55, 2464.
(b) Severino, E. A.; Correia, C. R. D. Org. Lett. 2000, 2, 3039.
(c) Oliveira, D. F.; Severino, E. A.; Correia, C. R. D. Tetrahedron Lett. 1999, 40, 2083.
(d) Severino, E. A.; Costenaro, E. R.; Garcia, A. L. L.; Correia, C. R. D. Org. Lett. 2003, 5, 305.
[24] For selected examples of enantioselective Heck reaction of five-membered tertiary enamides: (a) Hayashi, T.; Kubo, A.; Ozawa, F. Pure Appl. Chem. 1992, 64, 421.
(b) Loiseleur, O.; Hayashi, M.; Schmees, N.; Pfaltz, A. Synthesis 1997, 1338.
(c) Mazuela, J.; Pàmies, O.; Diéguez, M. Chem. Eur. J. 2010, 16, 3434.
(d) Hu, J.; Lu, Y.; Li, Y.; Zhou, J. Chem. Commun. 2013, 49, 9425.
(e) Wu, C.; Zhou, J. J. Am. Chem. Soc. 2014, 136, 650.
[25] Wheatley, B. M. M.; Keay, B. A. J. Org. Chem. 2007, 72, 7253.
[26] (a) Grigg, R.; Loganathan, V.; Santhakumar, V.; Sridharan, V.; Teasdale, A. Tetrahedron Lett. 1991, 132, 687.
(b) Ripa, L.; Hallberg, A. J. Org. Chem. 1997, 62, 595.
[27] Tu, T.; Hou, X.-L.; Dai, L.-X. Org. Lett. 2003, 5, 3651.
[28] Tietze, L. F.; Thede, K. Synlett 2000, 10, 1470.
[29] For selected examples of Heck reactions of 5-substituted five-membered tertiary enamides see 23(b) and 23(d).
[30] Oestreich, M. The Mizoroki-Heck Reaction, John Wiley & Sons, Ltd., 2009
[31] For details, see Table S1 in supporting information.
[32] For details, see Table S2 in supporting information.
[33] Crystallographic data of compound 4b and 4j see supporting information.
[34] Cabri, W.; Candiani, I. Acc. Chem. Res. 1995, 28, 2.
/
| 〈 |
|
〉 |