

Studies on a Novel Type of B(C6F5)3 and Aromatic Ammonium ChlorideSystem
Received date: 2015-07-04
Online published: 2015-08-18
Supported by
Project support by the Foundation of Sichuan Provincial Department of Education (No. 15ZA0279), Foundation of Leshan Science and Technology plan (No. 14GZD010)
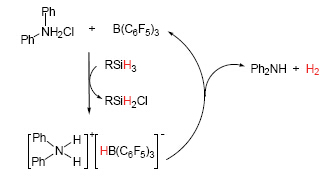
Although in recent years the frustrated Lewis pairs (FLPs) reactivity towards small molecule activation has been widely concerned, the reports on the FLPs derived from aromatic amines are few. This paper describes a new facile route to the aromatic amine based FLPs starting from aromatic amine hydrochloride/tri(pentafluorophenyl)borane (BCF) as a combined active couple and hydridosilane as a source of the hydride. The reaction characteristics have been studied for a set of the stoichiometrical reactions. The difference between the alkyl amine hydrochloride/BCF and aromatic amine hydrochloride/BCF systems is that after reacting with the silanes the formed aromatic ammonium hydridoborates [Ar2NH2]+[HBCF]- are not thermally stable and immediately release H2 with formation of the corresponding amine and BCF. However, it is precisely because of this fact making this system applicable for a catalytic reaction-a novel efficient chlorination reaction of hydridosilanes, which could be considered as the first example of the nonmetal catalytic reactions of the type, with the aromatic amine hydrochloride as a function of the chlorination reagent and BCF as a catalytic activator. The chlorination products and reaction mechanism have been also explored in detail under different ratios of silanes and aromatic amine hydrochlorides. Remarkably, if required, the initial aromatic amine hydrochloride can be regenerated and recycled by a simple reaction with gaseous HCl. This makes the reaction rather attractive from the viewpoint of “green chemistry”.
Tian Chong , Jiang Ya , Borzov Maxim , Nie Wanli . Studies on a Novel Type of B(C6F5)3 and Aromatic Ammonium ChlorideSystem[J]. Acta Chimica Sinica, 2015 , 73(11) : 1203 -1206 . DOI: 10.6023/A15070459
[1] Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
[2] Welch, G. C.; Stephan, D. W. J. Am. Chem. Soc. 2007, 129, 1880.
[3] Spies, P.; Erker, G.; Kehr, G.; Bergander, K.; Fraeohlich, R.; Grimme, S.; Stephan, D. W. Chem. Commun. 2007, 47, 5072.
[4] Chen, D. J.; Wang, Y.; Klankermayer, J. Angew. Chem., Int. Ed. 2010, 49, 9475.
[5] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46.
[6] Liu, Y.-B.; Du, H.-F. Acta Chim. Sinica 2014, 72, 771. (刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.)
[7] Feng, X.-Q.; Du, H.-F. Tetrahedron Lett. 2014, 55, 6959.
[8] Focante, F.; Mercandelli, P.; Sironi, A.; Resconi, L. Coord. Chem. Rev. 2006, 250, 170.
[9] Millot, N.; Santini, C. C.; Fenet, B.; Basset, J. M. Eur. J. Inorg. Chem. 2002, 3328.
[10] Sumerin, V.; Schulz, F.; Atsumi, M.; Wang, C.; Nieger, M.; Leskelae, M.; Repo, T.; Pyykkoe, P.; Rieger, B. J. Am. Chem. Soc. 2008, 130, 14117.
[11] Sumerin, V.; Schulz, F.; Nieger, M. Angew Chem., Int. Ed. 2008, 47, 6001.
[12] Schulz, F.; Sumerin, V.; Heikkinen, S.; Pedersen, B.; Wang, C.; Atsumi, M.; Leskelae, M.; Repo, T.; Pyykkoe, P.; Petry, W.; Rieger, B. J. Am. Chem. Soc. 2011, 133, 20245.
[13] Xu, Y.-Y.; Li, Z.; Borzov, M.; Nie, W.-L. Prog. Chem. 2012, 24(8), 1526. (徐莹莹, 李钊, Borzov Maxim, 聂万丽, 化学进展, 2012, 24(8), 1526.)
[14] Hu, X.; Tian, C.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1025. (胡茜, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1025.)
[15] Tian, C.; Borzov, M. V.; Liu, Q. CN104262374, 2015. [Chem. Abstr. 2015, 162, 219382]. (聂万丽, 田冲, Borzov, M. V., 刘芹, 专利申请号CN201410415316.7, 2014.)
[16] Nie, W.-L.; Tian, C.; Borzov, M. V.; Hu, X. CN104258904, 2015. [Chem. Abstr. 2015, 162, 209551]. (聂万丽, 田冲, Borzov, M. V.,胡茜, 专利申请号CN201410415003.1, 2014.)
[17] Nie, W.-L.; Tian, C.; Borzov, M. V.; Jiang, Y. CN104230975, 2014. [Chem. Abstr. 2014, 162, 123052]. (聂万丽, 田冲, Borzov, M. V.,姜亚, 专利申请号CN201410415290.6, 2014.)
[18] Roesler, R.; Piers, W. E.; Parvez, M. J. Organomet. Chem. 2003, 680, 218.
[19] Cunico, R. F.; Bedell, L. J. Org. Chem. 1980, 45, 4797.
[20] Khalafi-Nezhad, A.; Alamdari, R. F.; Zekri, N. Tetrahedron 2000, 56, 7503.
[21] Kunai, A.; Sakurai, T.; Toyoda, E.; Ishikawa, M. J. Organomet. Chem. 1996, 15, 2478.
[22] Kunai, A.; Ohshita, J. Organometallics 2003, 686, 3.
[23] Ferreri, C.; Costantino, C.; Romeo, R.; Chatgilialoglu, C. Tetrahedron Lett. 1999, 40, 1197.
/
| 〈 |
|
〉 |