

Recent Advances in the Synthesis of Distal Fluorinated Ketones
Received date: 2015-06-19
Online published: 2015-09-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21402134).
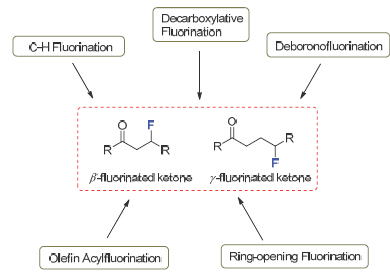
Incorporation of a fluorine atom to molecules is a privileged strategy to modify the physical, chemical and biological properties, which is widely executed in pharmaceuticals, agrochemical and material sciences. Consequently, the development of efficient and direct fluorination approach is of considerable significance. In spite of the great progress made in the transition-metal catalyzed construction of sp2 C—F bond via C—H activation and cross-coupling reactions, the direct access to aliphatic sp3 C—F bond is relatively underdeveloped as the development of a mild and efficient method to construct sp3 C—F bond remains a challenging issue. Ketone is a ubiquitous and important structural motif in organic compounds. The efficient synthesis of fluorinated ketone building blocks thus provides a shortcut for the introduction of fluorinated functionalities into complex molecules. Other than α-fluorinated ketones, the synthesis of distal fluorinated ketones is still challenging. Herein, we highlight the recent efforts made for the synthesis of distal fluorinated ketones. Four fluorination pathways are described: (a) C—H fluorination, (b) decarboxylative fluorination and deboronofluorination; (c) olefin fluorination, and (d) ring-opening fluorination. The first and second sections briefly introduce the direct sp3 C—H fluorination, decarboxylative fluorination, and deboronofluorination to construct aliphatic sp3 C—F bonds, which are tolerant to carbonyl group in the substrates. The third section discusses the direct construction of β and γ-fluorinated ketones via the difunctionalization of olefins. The last section puts an emphasis on the latest emergence of ring-opening fluorination. Relying on the “radical clock” strategy, the distal fluorinated ketones can be obtained from the corresponding tertiary cycloalkanols. Overall, this highlight provides a new insight into the recent advances in the sp3 C—H fluorination and the synthesis of distal fluorinated ketones.
Fan Xuefeng , Zhao Huijun , Zhu Chen . Recent Advances in the Synthesis of Distal Fluorinated Ketones[J]. Acta Chimica Sinica, 2015 , 73(10) : 979 -983 . DOI: 10.6023/A15060424
[1] (a) Hiyama, T. Organofluorine Compounds: Chemistry and Applications, Springer, New York, 2000.
(b) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity and Applications, 2nd ed., Wiley-VCH, Weinheim, 2013.
(c) Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, Eds.: Gouverneur, V.; Muller, K., Imperial College Press, London, 2012.
(d) Ni, C.; Zhu, L.; Hu, J. Acta Chim. Sinica 2015, 73, 90. (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90.)
(e) Zhu, H.; Liu, G. Acta Chim. Sinica 2012, 70, 2404. (朱海涛, 刘国生, 化学学报, 2012, 70, 2404.)
(f) Xiao, Y.; Pan, Q.; Zhang, X. Acta Chim. Sinica 2015, 73, 383. (肖玉兰, 潘强, 张新刚, 化学学报, 2015, 73, 383.)
[2] (a) Miller, P. W.; Long, N. J.; Vilar, R.; Gee, A. D. Angew. Chem., Int. Ed. 2008, 47, 8998.
(b) Ametamey, S. M.; Honer, M.; Schubiger, P. A. Chem. Rev. 2008, 108, 1501.
[3] For selected examples, see: (a) Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728.
(b) Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359.
(c) Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530.
(d) Ishimaru, T.; Shibata, N.; Horikawa, T.; Yasuda, N.; Nakamura, S.; Toru, T.; Shiro, M. Angew. Chem., Int. Ed. 2008, 47, 4157.
(e) Kwiatkowski, P.; Beeson, T. D.; Conrad, J. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2011, 133, 1738.
(f) Wang, L.; Meng, W.; Zhu, C.-L.; Zheng, Y.; Nie, J.; Ma, J.-A. Angew. Chem., Int. Ed. 2011, 50, 9442.
(g) Phipps, R. J.; Hiramatsu, K.; Toste, F. D. J. Am. Chem. Soc. 2012, 134, 8376.
[4] (a) Liu, W.; Groves, J. T. Angew. Chem., Int. Ed. 2013, 52, 6024.
(b) Huang, X.; Liu, W.; Ren, H.; Neelamegam, R.; Hooker, J. M.; Groves, J. T. J. Am. Chem. Soc. 2014, 136, 6842.
[5] (a) Bloom, S.; Pitts, C. R.; Woltornist, R.; Griswold, A.; Holl, M. G.; Lectka, T. Org. Lett. 2013, 15, 1722.
(b) Bloom, S.; Sharber, S. A.; Holl, M. G.; Knippel, J. L.; Lectka, T. J. Org. Chem. 2013, 78, 11082.
[6] Xia, J.; Zhu, C.; Chen, C. J. Am. Chem. Soc. 2013, 135, 17494.
[7] Kee, C. W.; Chin, K. F.; Wong, M. W.; Tan, C. H. Chem. Commun. 2014, 50, 8211.
[8] Yin, F.; Wang, Z.; Li, Z.; Li, C. J. Am. Chem. Soc. 2012, 134, 10401.
[9] Li, Z.; Wang, Z.; Zhu, L.; Tan, X.; Li, C. J. Am. Chem. Soc. 2014, 136, 16439.
[10] Wang, H.; Guo, L.; Duan, X. Chem. Commun. 2014, 50, 7382.
[11] Zhu, L.; Chen, H.; Wang, Z.; Li, C. Org. Chem. Front. 2014, 1, 1299.
[12] Zhao, H.; Fan, X.; Yu, J.; Zhu, C. J. Am. Chem. Soc. 2015, 137, 3490.
[13] Ishida, N.; Okumura, S.; Nakanishi, Y.; Murakami, M. Chem. Lett. 2015, 44, 821.
[14] Ren, S.; Feng, C.; Loh, T. Org. Biomol. Chem. 2015, 13, 5105.
[15] Bloom, S.; Bume, D. D.; Pitts, C. R.; Lectka, T. Chem. Eur. J. 2015, 21, 8060.
/
| 〈 |
|
〉 |