

Synthesis, Structure and Properties of a Novel Benzothiazole-based Diboron-Bridged π-Conjugated Ladder
Received date: 2015-09-14
Online published: 2015-10-12
Supported by
Project supported by the National Natural Science Foundation of China (No. 51173067).
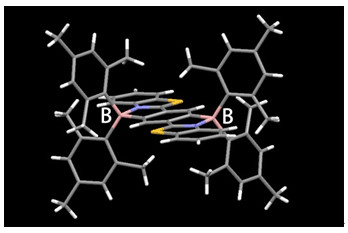
A novel diboron-bridged ladder-type molecule with extended π-conjugated skeleton has been designed and synthesized. Single crystal of the compound has been grown by the method of vacuum sublimation and the molecular structure determined by X-ray diffraction analysis demonstrate that this ladder-type molecule has a seven-ring fused skeleton, which is almost coplanar. And the two mesityl groups coordinated to each boron atom can effectively keep the luminescent units apart. No π-π interaction can be observed between the two extended π-conjugated planes. In the packing structures, we cannot find the intramolecular hydrogen bond, C-H…π interaction and other weak interaction. Based on UV-vis absorption and fluorescence emission spectra, the longest absorption band is peaked at 372 nm in dichloromethane solution and the emission band is at 544 nm which has a large stokes shift of 8499 cm-1. In the solid state, the compound shows yellow fluorescence with emission peak at 582 nm. The compound in condensed phase displays only slightly red shifted emission spectra and almost the same fluorescence quantum yield compared to that in dispersed phase, which is attributed to the bulky side groups on the boron atoms. The compound possesses a very high melting point (Tm=352℃) and decomposition temperature (Td5=360℃) due to the rigid π-conjugated plane that indicates its good thermal stability. The compound has two pairs of reversible reduction peaks and an irreversible oxidation peak which are similar to the reported four-coordinate compounds. The cyclic voltammogram curves indicate boron chelation can greatly lower the lowest unoccupied molecular orbital (LUMO). Thus, it makes cathodic reductions easier and thereby endows the π-conjugated ladder with enhanced electron-accepting nature. The electrochemical property suggests that the compound is suitable as an electron-transporting layer in organic light-emitting diode (OLED) devices. To obtain a deeper insight into the electronic structure and energy levels of the π-conjugated skeleton, density functional theory (DFT) calculations were performed. The LUMOs are delocalized on the entire seven-ring π-conjugated ladder while the central benzene ring and the mesityl chelated with the boron atom make a main contribution to the HOMOs. The general trend of the calculated HOMO/LUMO levels and energy gaps are basically consistent with the electrochemical and the photophysical data. Therefore, we fabricated OLEDs devices using the molecule as emitter and/or electron-transporting layers, which showed good EL performance.
Zhang Zhenyua , Zhang Zhenyua , Li Wanjun , Li Wanjun , Ye Kaiqi , Ye Kaiqi , Zhang Hongyu , Zhang Hongyu . Synthesis, Structure and Properties of a Novel Benzothiazole-based Diboron-Bridged π-Conjugated Ladder[J]. Acta Chimica Sinica, 2016 , 74(2) : 179 -184 . DOI: 10.6023/A15090603
[1] (a) Kawaguchi, K.; Nakano, K.; Nozaki, K. J. Org. Chem. 2007, 72, 5119.
(b) Anthony, J. E. Chem. Rev. 2006, 106, 5028.
(c) Bendikov, M.; Wudl, F.; Perepichka, D. F. Chem. Rev. 2004, 104, 4891.
(d) Xiao, K.; Liu, Y.; Qi, T.; Zhang, W.; Wang, F.; Gao, J.; Qiu, W.; Ma, Y.; Cui, G.; Chen, S.; Zhan, X.; Yu, G.; Qin, J.; Hu, W.; Zhu, D. J. Am. Chem. Soc. 2005, 127, 13281.
(e) Fukazawa, A.; Yamaguchi, S. Chemistry-An Asian Journal 2009, 4, 1386.
[2] (a) Liu, Z.-Q.; Fang, Q.; Wang, D.; Xue, G.; Cao, D.-X.; Yu, W.-T.; Xia, G.-M. Acta Chim. Sinica 2003, 61, 1449. (刘志强, 方奇, 王东, 薛刚, 曹笃霞, 于文涛, 夏光明, 化学学报, 2003, 61, 1449.)
(b) Wang. W.; Fang, Q.; Liu, Z.-Q.; Cao, D.-X.; Deng, M.-Z. Acta Chim. Sinica 2005, 63, 1323. (王伟, 方奇, 刘志强, 曹笃霞, 邓敏智, 化学学报, 2005, 63, 1415.)
(c) Cao, D.-X.; Liu, Z.-Q.; Wang, D.; Fang, Q. Acta Chim. Sinica 2005, 63, 1415. (曹笃霞, 刘志强, 王东, 方奇, 化学学报, 2005, 63, 1415.)
(d) Li, X.-H.; Xu, B.; Lv, K.-L.; Zhang, A.-Q. Chin. Chem. Lett. 2011, 22, 599.
[3] (a) Rao, Y.-L.; Wang, S. Inorg. Chem. 2011, 50, 12263.
(b) Li, D.; Zhang, H.; Wang, Y. Chem. Soc. Rev. 2013, 42, 8416.
(c) Zhang, H.; Huo, C.; Ye, K.; Zhang, P.; Tian, W.; Wang, Y. Inorg. Chem. 2006, 45, 2788.
(d) Zhang, H.; Huo, C.; Zhang, J.; Zhang, P.; Tian, W.; Wang, Y. Chem. Commun. 2006, 281.
(e) Zhang, Z.; Yao, D.; Zhao, S.; Gao, H.; Fan, Y.; Su, Z.; Zhang, H.; Wang, Y. Dalton Trans. 2010, 39, 5123.
(f) Chen, H. Y.; Chi, Y.; Liu, C. S.; Yu, J. K.; Cheng, Y. M.; Chen, K. S.; Chou, P. T.; Peng, S. M.; Lee, G. H.; Carty, A. J.; Yeh, S. J.; Chen, C. T. Adv. Funct. Mater. 2005, 15, 567.
(g) Liu, Q. D.; Mudadu, M. S.; Thummel, R.; Tao, Y.; Wang, S. Adv. Funct. Mater. 2005, 15, 143.
[4] (a) Kubo, Y.; Watanabe, K.; Nishiyabu, R.; Hata, R.; Murakami, A.; Shoda, T.; Ota, H. Org. Lett. 2011, 13, 4574.
(b) Chen, J. J.; Conron, S. M.; Erwin, P.; Dimitriou, M.; McAlahney, K.; Thompson, M. E. ACS Appl. Mater. 2015, 7, 662.
(c) Yoshii, R.; Yamane, H.; Nagai, A.; Tanaka, K.; Taka, H.; Kita, H.; Chujo, Y. Macromolecules 2014, 47, 2316.
(d) Min, J.; Ameri, T.; Gresser, R.; Lorenz-Rothe, M.; Baran, D.; Troeger, A.; Sgobba, V.; Leo, K.; Riede, M.; Guldi, D. M.; Brabec, C. J. ACS Appl. Mater. 2013, 5, 5609.
[5] Wang, L.; Zhang, Z.; Cheng, X.; Ye, K.; Li, F.; Wang, Y.; Zhang, H. J. Mater. Chem. C 2015, 3, 499.
[6] (a) Boens, N.; Leen, V.; Dehaen, W. Chem. Soc. Rev. 2012, 41, 1130.
(b) Madhu, S.; Ravikanth, M. Inorg. Chem. 2012, 51, 4285.
(c) Lifschitz, A. M.; Shade, C. M.; Spokoyny, A. M.; Mendez-Arroyo, J.; Stern, C. L.; Sarjeant, A. A.; Mirkin, C. A. Inorg. Chem. 2013, 52, 5484.
(d) Sarkar, S. K.; Mukherjee, S.; Thilagar, P. Inorg. Chem. 2014, 53, 2343.
(e) Tian, M.-Z.; Feng, F.; Meng, S.-M.; Yuan, Y.-H. Chinese Chem. Lett. 2009, 20, 326.
[7] (a) Cui, Y.; Liu, Q.-D.; Bai, D.-R.; Jia, W.-L.; Tao, Y.; Wang, S. Inorg. Chem. 2005, 44, 601.
(b) Zhang, Z.; Zhang, H.; Jiao, C.; Ye, K.; Zhang, H.; Zhang, J.; Wang, Y. Inorg. Chem. 2015, 54, 2652.
[8] (a) Liddle, B. J.; Silva, R. M.; Morin, T. J.; Macedo, F. P.; Shukla, R.; Lindeman, S. V.; Gardinier, J. R. J. Org. Chem. 2007, 72, 5637;
(b) Xu, C.-Y.; Huang, K.-J.; Xie, W.-Z. Acta Chim. Sinica 2009, 67, 1075. (许春莹, 黄克靖, 谢宛珍, 化学学报, 2009, 67, 1075.)
(c) He, L.-Y.; Hu, Q.-Z.; Xi, H.-T.; Sun, X.-Q. Chin. J. Org. Chem. 2015, 35, 232(in Chinese). (贺琳彦, 胡全子, 席海涛, 孙小强, 有机化学, 2015, 35, 232.)
(d) He, Y.; Feng, R.-K.; Yi, Y.-R.; Liu, Z.-X. Chin. J. Org. Chem. 2014, 34, 2236. (何源, 冯若昆, 易云瑞, 刘占祥, 有机化学, 2014, 34, 2236.)
(e) Ye, J.-H.; Ye, W.-F.; Xiao, C.-T.; Chen, Y.; Wang, G.-P.; Zhang, W.-C. Chin. J. Org. Chem. 2012, 32, 1503. (叶家海, 叶文芳, 肖承涛, 陈雨, 王光普, 张文超, 有机化学, 2012, 32, 1503.)
(f) Lei, Y.-P.; Cao, S.-W. Chin. Chem. Lett. 2010, 21, 135.
[9] (a) Son, H.-J.; Han, W.-S.; Wee, K.-R.; Chun, J.-Y.; Choi, K.-B.; Han, S. J.; Kwon, S.-N.; Ko, J.; Lee, C.; Kang, S. O. Eur. J. Inorg. Chem. 2009, 2009, 1503.
(b) Job, A.; Wakamiya, A.; Kehr, G.; Erker, G.; Yamaguchi, S. Org. Lett. 2010, 12, 5470.
(c) Matsuo, K.; Saito, S.; Yamaguchi, S. J. Am. Chem. Soc. 2014, 136, 12580.
[10] (a) Li, D.; Zhang, Z.; Zhao, S.; Wang, Y.; Zhang, H. Dalton Trans. 2011, 40, 1279.
(b) Li, D.; Wang, K.; Huang, S.; Qu, S.; Liu, X.; Zhu, Q.; Zhang, H.; Wang, Y. J. Mater. Chem. 2011, 21, 15298.
(c) Li, D.; Yuan, Y.; Bi, H.; Yao, D.; Zhao, X.; Tian, W.; Wang, Y.; Zhang, H. Inorg. Chem. 2011, 50, 4825.
(d) Zhang, Z.; Bi, H.; Zhang, Y.; Yao, D.; Gao, H.; Fan, Y.; Zhang, H.; Wang, Y.; Wang, Y.; Chen, Z.; Ma, D. Inorg. Chem. 2009, 48, 7230.
[11] (a) Iida, A.; Yamaguchi, S. J. Am. Chem. Soc. 2011, 133, 6952.
(b) Fukazawa, A.; Yamaguchi, E.; Ito, E.; Yamada, H.; Wang, J.; Irle, S.; Yamaguchi, S. Organometallics 2011, 30, 3870.
[12] Curiel, D.; Más-Montoya, M.; Usea, L.; Espinosa, A.; Orenes, R. A.; Molina, P. Org. Lett. 2012, 14, 3360.
[13] (a) Yamaguchi, E.; Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Sasaki, T.; Ueda, M.; Sasaki, N.; Higashiyama, T.; Yamaguchi, S. Angew. Chem. Int. Ed. 2015, 54, 4539.
(b) Zhang, D.; Wen, Y.; Xiao, Y.; Yu, G.; Liu, Y.; Qian, X. Chem. Commun. 2008, 4777.
(c) Hu, R.; Gomez-Duran, C. F. A.; Lam, J. W. Y.; Belmonte-Vazquez, J. L.; Deng, C.; Chen, S.; Ye, R.; Pena-Cabrera, E.; Zhong, Y.; Wong, K. S.; Tang, B. Z. Chem. Commun. 2012, 48, 10099.
(d) Mao, M.; Xiao, S.; Li, J.; Zou, Y.; Zhang, R.; Pan, J.; Dan, F.; Zou, K.; Yi, T. Tetrahedron 2012, 68, 5037.
[14] (a) SHELXTL, Version 5.1; Siemens Industrial Automation, Inc. 1997.
(b) Sheldrick, G. M., SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997.
[15] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision C.02, Gaussian, Inc., Pittsburgh, PA, 2003.
[16] Runge, E.; Gross, E. K. U. Phys. Rev. Lett. 1984, 52, 997.
/
| 〈 |
|
〉 |