

Synthesis, Mechanochromism and Acid Response of the Fluorescence Dyes Based on Quinoxalines Modified with Tetraphenylethylenes
Received date: 2015-09-25
Online published: 2015-10-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 21374041) and the Open Project of State Key Laboratory of Supramolecular Structure and Materials (No. SKLSSM2015014).
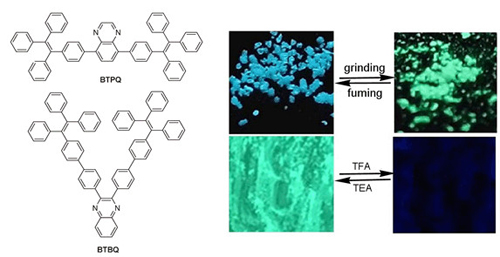
Three new D-π-A type quinoxalines modified with tetraphenylethylenes BTPQ, DBTPQ and BTBQ were synthesized via Suzuki coupling reactions between (4-(1,2,2-triphenylvinyl)phenyl)boronic acid and bromo aromatic hydrocarbons. It was found that BTPQ and DBTPQ, in which tetraphenylethylenes were substituted on 5,8-positions of quinoxalines gave the absortion bands at 316 nm and 303 nm, respectively, originated from π-π* transition. For BTBQ, in which tetraphenylethylene units were located at 2,3-positions of quinoxaline, the π-π* transition absorption blue-shifted to 287 nm on account of the poor planarity and low conjugation. Meanwhile, the intermolecular charge transfer (ICT) emission could be detected for BTPQ and DBTPQ, whose emission bands red-shifted significantly and emission intensities decreased with increasing the solvent polarities. It should be noted that the three compounds exhibited aggregation-induced emission (AIE) behaviors. For instance, when the water faction in the THF solution increased to 90%, the emission intensity at ca. 400 nm for BTBQ, was ca. 54 times higher than that in THF. Additionally, trifluoroacetic acid (TFA) could lead to the changes of color and emitting color of BTBQ in solution as well as in solid state due to the formation of protonated quinoxaline. We found that the grey solid of BTBQ could turn into red one upon exposed to gaseous TFA, accompanying with the quench of the emission. Other kinds of acids of HCl, HNO3 and acetic acid also could lead to the fluorescence quenching of solid BTBQ to some extent. Therefore, BTBQ could be used as sensory material to detect acid vapors by naked eyes. However, the protonation would be prohibited in BTPQ and DBTPQ on account of the steric effect of tetraphenylethylene units linked to 5,8-positions of quinoxaline, so BTPQ and DBTPQ could not detect acid. Interestingly, the solid emitting colors of BTPQ as well as DBTPQ were quite different before and after grinding, exhibiting mechanochromic properties. The as-prepared crystal of BTPQ emitting blue light under UV irradiation could be changed into amorphous powder with bluish green emission. The XRD patterns suggested that the mechanochromism was originated from the transition between the crystalline and amorphous states. Such mechanochromism was reversible under the treatment of grinding and heating/fuming with DCM.
Key words: quinoxaline; tetraphenylethylene; mechanochromism; acid response; AIE
Sun Jingbo , Sun Jingbo , Zhang Gonghe , Zhang Gonghe , Jia Xiaoyu , Jia Xiaoyu , Xue Pengchong , Xue Pengchong , Jia Junhui , Jia Junhui , Lu Ran , Lu Ran . Synthesis, Mechanochromism and Acid Response of the Fluorescence Dyes Based on Quinoxalines Modified with Tetraphenylethylenes[J]. Acta Chimica Sinica, 2016 , 74(2) : 165 -171 . DOI: 10.6023/A15090628
[1] (a) Sagara, Y.; Kato, T. Nat. Chem. 2009, 1, 605;
(b) Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. J. Chem. Soc. Rev. 2012, 41, 3878;
(c) Yao, X. D.; Chi, Z. G. Sci. China Chem. 2013, 43, 1090. (姚献东, 池振国, 中国科学, 2013, 43, 1090.)
[2] Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 1740.
[3] (a) Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361;
(b) Zhao, Z. J.; Lam, J. W. Y.; Tang, B. Z. Soft Matter 2013, 9, 4564.
[4] (a) Peng, B. Y.; Xu, S. D.; Chi, Z. G.; Zhang, X. Q.; Zhang, Y.; Xu, J. R. Prog. Chem. 2003, 25, 1085. (彭邦银, 许适当, 池振国, 张锡奇, 张艺, 许家瑞, 化学进展, 2003, 25, 1085);
(b) Huang, T.; Wang, Z. Y.; Qin, A. J.; Sun, J. Z.; Tang, B. Z. Acta Chim. Sinica 2013, 71, 979. (黄田, 汪昭旸, 秦安军, 孙景志, 唐本忠, 化学学报, 2013, 71, 979.)
[5] (a) Misra, R.; Jadhav, T.; Dhokale, B.; Mobin, S. M. Chem. Commun. 2014, 50, 9076; (b) Luo, X.; Li, J.; Li, C.; Heng, L.; Dong, Y.; Liu, Z.; Bo, Z.; Tang, B. Adv Mater. 2011, 23, 3261;
(c) Wang, J.; Mei, J.; Hu, R.; Sun, J.; Qin, A.; Tang, B. J. Am. Chem. Soc. 2012, 134, 9956;
(d) Li, H.; Chi, Z.; Xu, B.; Zhang, X.; Li, X.; Liu, S.; Zhang, Y.; Xu, J. J. Mater. Chem. 2011, 21, 3760;
(e) Zhou, X.; Li, H.; Chi, Z.; Zhang, X.; Zhang, J.; Xu, B.; Zhang, Y.; Liu, S.; Xu, J. New J. Chem. 2012, 36, 685.
[6] (a) Wang, Y.; Liu, W.; Bu, L.; Li, J.; Zheng, M.; Zhang, D.; Sun, M.; Tao, Y.; Xue, S.; Yang, W. J. Mater. Chem. C 2013, 1, 856;
(b) Yagai, S.; Seki, T.; Kitamura, T.; Würthner, F. Angew. Chem., Int. Ed. 2008, 47, 3367;
(c) Dong, Y.; Xu, B.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Lv, H.; Wen, S.; Li, B.; Ye, L.; Zou, B.; Tian, W. J. Angew. Chem. Int. Ed. 2012, 51, 10782.
[7] Gong, Y.; Tan, Y.; Liu, J.; Lu, P.; Feng, C.; Yuan, W.; Lu, Y.; Sun, J.; He, G.; Zhang, Y. Chem. Commun. 2013, 49, 4009.
[8] (a) An, B. K.; Kwon, S. K.; Jung, S. D.; Park, S. Y. J. Am. Chem. Soc. 2002, 124, 14410;
(b) Yuan, W. Z.; Tan, Y.; Gong, Y.; Lu, P.; Lam, J. W. Y.; Shen, X.; Feng, C.; Sung, H. H. Y.; Lu, Y.; Williams, I. D.; Sun, J.; Zhang, Y.; Tang, B. Adv. Mater. 2013, 25, 2837;
(c) Gong, Y.; Liu, J.; Zhang, Y.; He, G.; Lu, Y.; Fan, W. B.; Yuan, W. Z.; Sun, J. Z.; Zhang, Y. J. Mater. Chem. C 2014, 2, 7552.
[9] (a) Sagara, Y.; Mutai, T.; Yoshikawa, I.; Araki, K. J. Am. Chem. Soc. 2007, 129, 1520; (b) Yamane, S.; Sagara, Y.; Mutai, T.; Araki, K.; Kato, T. J. Mater. Chem. C 2013, 1, 2648.
[10] (a) Shen, X. Y.; Yuan, W. Z.; Liu, Y.; Zhao, Q.; Lu, P.; Ma, Y.; Williams, I. D.; Qin, A.; Sun, J. Z.; Tang, B. Z. J. Phys. Chem. C 2012, 116, 10541;
(b) Shen, X. Y.; Wang, Y. J.; Zhao, E.; Yuan, W. Z.; Liu, Y.; Lu, P.; Qin, A.; Ma, Y.; Sun, J. Z.; Tang, B. Z. J. Phys. Chem. C 2013, 117, 7334;
(c) Kim, F. S.; Guo, X.; Watson, M. D.; Jenekhe, S. A. Adv. Mater. 2010, 22, 478;
(e) Lin, T. C.; He, G.; Zheng, S. Q. J. Mater. Chem. 2006, 16, 2490.
[11] (a) Ooyama, Y.; Ito, G.; Fukuoka, H.; Nagano, T.; Kagawa, Y.; Imae, I.; Komaguchi, K.; Harima, Y. Tetrahedron 2010, 66, 7268; (b) Ooyama, Y.; Harima, Y. J. Mater. Chem. 2011, 21, 8372.
[12] (a) Xue, P. C.; Yao, B. Q.; Sun, J. B.; Xu, Q. X.; Chen, P.; Zhang, Z. Q.; Lu, R. J. Mater. Chem. C 2014, 2, 3942;
(b) Xue, P. C.; Chen, P.; Jia, J. H.; Xu, Q. X.; Sun, J. B.; Yao, B. Q.; Zhang, Z. Q.; Lu, R. Chem. Commun. 2014, 50, 2569;
(c) Zhang, Z. Q.; Xue, P. C.; Gong, P.; Zhang, G. H.; Peng, J.; Lu, R. J. Mater. Chem. C 2014, 2, 9543;
(d) Xue, P. C.; Yao, B. Q.; Wang, P. P.; Sun, J. B.; Zhang, Z. Q.; Lu, R. RSC Adv. 2014, 4, 58732.
[13] (a) Xue, P. C.; Yao, B. Q.; Liu, X. H.; Sun, J. B.; Gong, P.; Zhang, Z. Q.; Qian, C.; Zhang, Y.; Lu, R. J. Mater. Chem. C 2015, 3, 1018;
(b) Zhang, Z. Q.; Wu, Z.; Sun, J. B.; Yao, B. Q.; Zhang, G. H.; Xue, P. C.; Lu, R. J. Mater. Chem. C 2015, 3, 4921;
(c) Zhang, G. H.; Sun, J. B.; Xue, P. C.; Zhang, Z. Q.; Gong, P.; Peng, J.; Lu, R. J. Mater. Chem. C 2015, 3, 2925.
[14] Muniz, F. M.; Alcazar, V.; Sanz, F.; Simon, L.; de Arriba, A. L. F.; Raposo, C.; Moran, J. R. Eur. J. Org. Chem. 2010, 6179.
[15] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A.; Bloino, F. J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery Jr, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J. M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A. 02, Gaussian, Inc., Wallingford, CT, 2009.
[16] Chi, Z. G.; He, K. Q.; Li, H. Y.; Zhang, X. Q.; Xu, B. J.; Liu, S. W.; Zhang, Y.; Xu, J. R. Chem. J. Chin. Univ. 2012, 33, 725. (池振国, 何克强, 李海银, 张锡奇, 许炳佳, 刘四委, 张艺, 许家瑞, 高等学校化学学报, 2012, 33, 725.)
[17] Vasanthan, N.; Manne, J.; Krishnama, A. Ind. Eng. Chem. Res. 2013, 52, 17920.
/
| 〈 |
|
〉 |