

Synthesis and Photovoltaic Performance of Water/Alcohol Soluble Small Phorphyrin Derivatives for Polymer Solar Cells
Received date: 2015-08-16
Online published: 2015-11-19
Supported by
Project supported by the Ministry of Science and Technology (No. 2014CB643501), the National Natural Science Foundation of China (Nos. 51303056, 21125419, 21490573 and 51361165301) and the Natural Science Foundation of Guangdong Province (No. S2012030006232).
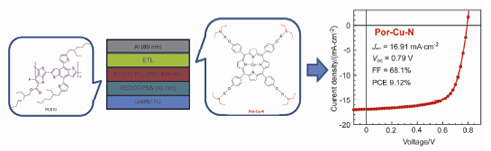
A series of novel water/alcohol soluble small molecular phorphyrin derivatives, Por-N, Por-NBr, Por-Cu-N and Por-Cu-NBr, were designed and synthesized. Differential scanning calorimetry measurements indicated that the neutral molecules Por-N and Por-Cu-N exhibited distinct melting point and crystallinity, while no observable characteristics were realized for the quaternized molecules. All these small molecules exhibited good thermal stability as evaluated by thermal gravimetric analysis. The photophysical properties of these small molecules were systematically investigated by UV-vis spectroscopy in both solutions and as thin films. It was found that the absorption spectra of Por-Cu-N and Por-Cu-NBr were slightly blue-shifted due to the formation of complex between porphyrin and copper. The highest occupied molecular orbitals energy levels showed negligible changes as determined by cyclic voltammetry measurements. The bulk-heterojunction solar cells utilizing these phorphyrin derivatives as the cathode interlayer and PCE10:PC71BM as the photoactive layer were fabricated. The single carrier devices revealed that the electron mobility of these phorphyrin derivatives are much higher than the hole-mobility except Por-N. Polymer solar cell devices with the structure of ITO/PEDOT:PSS/PCE10: PC71BM/Porphyrin derivatives/Al were fabricated to evaluate the function of cathode interlayer of these molecules. It was also worth noting that the polymer solar cell devices by using these molecules as the cathode interlayer exhibited obviously improved performances than the methanol treated device. Devices based on the molecules of Por-NBr, Por-Cu-N and Por-Cu-NBr as the cathode interlayer and narrow bandgap PCE10:PC71BM blend film as the photoactive layer exhibited comparatively high power conversion efficiency exceeding 9%. The best power conversion efficiency of 9.12% was achieved by the device based on Por-Cu-N as the cathode inter layer, with corresponding parameters of short circuit current of 16.91 mA·cm-2, open circuit voltage of 0.79 V and fill factor of 68.1%. These observations demonstrated that the synthesized water/alcohol soluble small molecules based on porphyrin derivatives can be prominent cathode interfacial layers for polymer solar cells.
Lu Junming , Cai Wanqing , Zhang Guichuan , Liu Shengjian , Ying Lei , Huang Fei . Synthesis and Photovoltaic Performance of Water/Alcohol Soluble Small Phorphyrin Derivatives for Polymer Solar Cells[J]. Acta Chimica Sinica, 2015 , 73(11) : 1153 -1160 . DOI: 10.6023/A15080546
[1] Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Nat. Commun. 2014, 5, 5293.
[2] He, Z. C.; Xiao, B.; Liu, F.; Wu, H. B.; Yang, Y. L.; Xiao, S.; Wang, C.; Russell, T. P.; Cao, Y. Nat. Photon. 2014, 9, 174.
[3] Zhang, S. Q.; Ye, L.; Zhao, W. C.; Yang, B.; Wang, Q.; Hou, J. H. Sci China, Chem. 2015, 58, 248.
[4] Vohra, V.; Kawashima, K.; Kakara, T.; Koganezawa, T.; Osaka, I.; Takimiya, K.; Murata, H. Nat. Photon. 2015, 9, 403.
[5] Huo, L. J.; Hou, J. H. Sci China, Chem. 2012, 42, 702. (霍利军, 侯剑辉, 中国科学:化学, 2012, 42, 702.)
[6] Cheng, P.; Shi, Q. Q.; Zhan, X. W. Acta Chim. Sinica 2015, 73, 252. (程沛, 史钦钦, 占肖卫, 化学学报, 2015, 73, 252.)
[7] Chen, Y. S.; Wan, X. J.; Long, G. K. Acc. Chem. Res. 2013, 46, 2645.
[8] Li, Y. F. Acc. Chem. Res. 2012, 45, 723.
[9] Ye, L.; Zhang, S. Q.; Huo, L. J.; Zhang, M. J.; Hou, J. H. Acc. Chem. Res. 2014, 47, 1595.
[10] Li, G.; Zhu, R.; Yang, Y. Nat. Photon. 2012, 6, 153.
[11] Liu, S. J.; Zhang, K.; Lu, J. M.; Zhang, J.; Yip, H. L.; Huang, F.; Cao, Y. J. Am. Chem. Soc. 2013, 135, 15326.
[12] Hu, Z. C.; Zhang, K.; Huang, F.; Cao, Y. Chem. Commun. 2015, 51, 5572.
[13] Zhang, K.; Hu, Z. C.; Xu, R. G.; Jiang, X. F.; Yip, H.-L.; Huang, F.; Cao, Y. Adv. Mater. 2015, 27, 3607.
[14] Seo, J. H.; Gutacker, A.; Sun, Y. M.; Wu, H. B.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A. J.; Bazan, G. C. J. Am. Chem. Soc. 2011, 133, 8416.
[15] Zhang, K.; Guan, X.; Huang, F.; Cao, Y. Acta Chim. Sinica 2012, 73, 252. (张凯, 管星, 黄飞, 曹镛, 化学学报, 2012, 73, 252.)
[16] Zhang, K.; Zhong, C. M.; Liu, S. J.; Mu, C.; Li, Z. K.; Yan, H.; Huang, F.; Cao, Y. ACS Appl. Mater. Interfaces 2014, 6, 10429.
[17] Kumar, C. V.; Cabau, L.; Koukaras, E. N.; Sharma, G. D.; Palomares, E. Nanoscale 2015, 7, 179.
[18] Qin, H. M.; Li, L. S.; Guo, F.; Su, S. J.; Peng, J. B.; Cao, Y.; Peng, X. B. Energy Environ. Sci. 2014, 7, 1397.
[19] Chen, S.; Xiao, L. G.; Zhu, X. L.; Peng, X. B.; Wong, W.-K.; Wong, W.-Y. Chem. Commun. 2015, 51, 14439.
[20] Gao, K.; Li, L. S.; Lai, T. Q.; Xiao, L. G.; Huang, Y.; Huang, F.; Peng, J. B.; Cao, Y.; Liu, F.; Russell, T. P.; Janssen, R. A.; Peng, X. B. J. Am. Chem. Soc. 2015, 137, 7282.
/
| 〈 |
|
〉 |