

Targeted Syntheses of Charged Porous Aromatic Frameworks for Iodine Enrichment and Release
Received date: 2015-08-25
Online published: 2015-11-24
Supported by
Project supported by National Basic Research Program of China (973 Program, No. 2012CB821700), Major International (Regional) Joint Research Project of NSFC (No. 21120102034) and NSFC (No. 20831002).
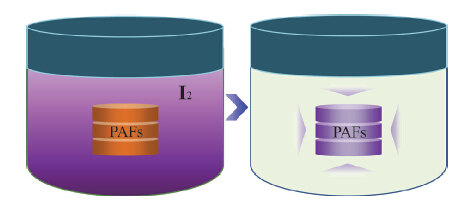
In this paper, we synthesized two charged porous aromatic frameworks (PAF-21 and PAF-22) using lithium tetrakis(4-iodophenyl)borate as tetrahedral units and 1,4-benzenediboronic acid or 4,4'-biphenyldiboronic acid as linear linkers via a Suzuki coupling reaction. FTIR spectra prove the completion of the coupling reaction. The absence of the B-OH band (at 3370 cm-1) and the C-I bands (at 480 and 506 cm-1) in the FTIR spectra indicate the formation of polymeric networks. The solid-state 13C CP/MAS NMR spectra of PAF-21 and PAF-22 showed the carbon resonances with chemical shift in the range of δ 120~145, which are related to aromatic carbon atoms of framework-building phenylene groups. Simultaneously, the only resonance at δ -26 shown in 11B MAS NMR spectra can be attributed to the central B atom in the framework. Both 13C CP/MAS NMR and 11B MAS NMR analyses show the absence of other resonances, testifying that an almost complete coupling reaction has taken place. Powder X-ray diffraction (PXRD) of these PAFs revealed their amorphous texture, no long-range ordered frameworks could be detected due to the distortion and interpenetration of the phenyl rings. Scanning electron microscopy (SEM) images showed that PAF-21 and PAF-22 were composed of large fused polymer masses. Transmission electron microscopy (TEM) images also revealed that they were amorphous materials. Thermogravimetric analysis (TGA) showed that PAF-21 and PAF-22 were thermally stable up to 350 °C under atmosphere. In addition, these materials also exhibit high chemical stability, as verified by no dissolution or decomposition in common organic solvents such as methanol, ethanol, acetone, THF, CH2Cl2, CHCl3, DMF, etc. Carbon dioxide sorption isotherms were measured on the activated samples at 273 K and 1 bar. The CO2 uptake is 19.2 mg/g for PAF-21 and 22.5 mg/g for PAF-22, respectively. Compared with other materials such as zeolites and metal-organic frameworks, these PAFs show very high affinity and capacity for iodine (1520 mg/g for PAF-21 and 1960 mg/g for PAF-22 respectively) due to their special charged aromatic networks. Significantly, PAF-21 and PAF-22 could reversibly release iodine molecules in ethanol solution, might be used in practical and commercial applications for contaminative iodine treatment.
Yan Zhuojun , Yuan Ye , Liu Jia , Li Qin , Nguyen Nam-Trung , Zhang Daming , Tian Yuyang , Zhu Guangshan . Targeted Syntheses of Charged Porous Aromatic Frameworks for Iodine Enrichment and Release[J]. Acta Chimica Sinica, 2016 , 74(1) : 67 -73 . DOI: 10.6023/A15080562
[1] Vienna, J. D. Int. J. Appl. Glass Sci. 2010, 1, 309.
[2] Kintisch, E. Science 2005, 310, 1406.
[3] Ewing, R. C.; von Hippel, F. N. Science 2009, 325, 151.
[4] Ojovan, M. I.; Lee, W. E. An Introduction to Nuclear Waste Immobilisation, Elsevier Science, Amsterdam, 2005.
[5] Jubin, R. T. Organic Iodine Removal from Simulated Dissolver Off-Gas Streams Using Silver Exchanged Mordenite, In Proceedings of the 16th DOE Nuclear Air Cleaning Conference, 1981, Paper No. CONF-8208322.
[6] Haefner, D. R.; Tranter, T. J. Methods of Gas Phase Capture of Iodine from Fuel Reprocessing Off-Gas: A Literature Survey, INL/EXT-07-12299, Idaho National Laboratory: Idaho Falls, ID, 2007.
[7] Hertzsch, T.; Budde, F.; Weber, E.; Hülliger, J. Angew. Chem., Int. Ed. 2002, 41, 2281.
[8] Chapman, K. W.; Chupas, P. J.; Nenoff, T. M. J. Am. Chem. Soc. 2010, 132, 8897.
[9] Wang, Z. M.; Zhang, B.; Fujiwara, H.; Kobayashi, H.; Kurmoo, M. Chem. Commun. 2004, 416.
[10] Choi, H. J.; Suh, M. P. J. Am. Chem. Soc. 2004, 126, 15844.
[11] Abrahams, B. F.; Moylan, M.; Orchard, S. D.; Robson, R. Angew. Chem., Int. Ed. 2003, 42, 1848.
[12] Dobrzańska, L.; Lioyd, G. O.; Raubenheimer, H. G.; Barbour, L. J. J. Am. Chem. Soc. 2006, 128, 698.
[13] Zeng, M. H.; Wang, Q. X.; Tan, Y. X.; Hu, S.; Zhao, H. X.; Long, L. S.; Kurmoo, M. J. Am. Chem. Soc. 2010, 132, 2561.
[14] Wang, Z. M.; Zhang, Y. J.; Liu, T.; Kurmoo, M.; Gao, S. Adv. Funct. Mater. 2007, 17, 1523.
[15] Sava, D. F.; Rodriguez, M. A.; Chapman, K. W.; Chupas, P. J.; Greathouse, J. A.; Crozier, P. S.; Nenoff, T. M. J. Am. Chem. Soc. 2011, 133, 12398.
[16] Sava, D. F.; Chapman, K. W.; Rodriguez, M. A.; Greathouse, J. A.; Crozier, P. S.; Zhao, H. Y.; Chupas, P. J.; Nenoff, T. M. Chem. Mater. 2013, 25, 2591.
[17] Kitagawa, H.; Ohtsu, H.; Kawano, M. Angew. Chem., Int. Ed. 2013, 52, 12395.
[18] Katsoulidis, A. P.; He, J. Q.; Kanatzidis, M. G. Chem. Mater. 2012, 24, 1937.
[19] Pei, C. Y.; Ben, T.; Xu, S. X.; Qiu, S. L. J. Mater. Chem. A 2014, 2, 7179.
[20] Hasell, T.; Schmidtmann, M.; Cooper, A. I. J. Am. Chem. Soc. 2011, 133, 14920.
[21] Sigen, A.; Zhang, Y. W.; Li, Z. P.; Xia, H.; Xue, M.; Liu, X. M.; Mu, Y. Chem. Commun. 2014, 50, 8495.
[22] Holst, J. R.; Stöckel, E.; Adams, D. J.; Cooper, A. I. Macromolecules 2010, 43, 8531.
[23] Stockel, E.; Wu, X. F.; Trewin, A.; Wood, C. D.; Clowes, R.; Campbell, N. L.; Jones, J. T. A.; Khimyak, Y. Z.; Adams, D. J.; Cooper, A. I. Chem. Commun. 2009, 212.
[24] Yuan, Y.; Yan, Z. J.; Ren, H.; Liu, Q. Y.; Zhu, G. S.; Sun, F. X. Acta Chim. Sinica 2012, 70, 1446. (元野, 闫卓君, 任浩, 刘青英, 朱广山, 孙福兴, 化学学报, 2012, 70, 1446.)
[25] Zhang, T. T.; Wang, H. T.; Ma, H. P.; Sun, F. X.; Cui, X. Q.; Zhu, G. S. Acta Chim. Sinica 2013, 71, 1598. (张婷婷, 王海涛, 马和平, 孙福兴, 崔小强, 朱广山, 化学学报, 2013, 71, 1598.)
[26] Wang, W.; Yan, Z. J.; Yuan, Y.; Sun, F. X.; Zhao, M.; Ren, H.; Zhu, G. S. Acta Chim. Sinica 2014, 72, 557. (王维, 闫卓君, 元野, 孙福星, 赵明, 任浩, 朱广山, 化学学报, 2014, 72, 557.)
[27] Song, W. C.; Xu, X. K.; Chen, Q.; Zhuang, Z. Z.; Bu, X. H. Polym. Chem. 2013, 4, 4690.
[28] Chang, Z.; Zhang, D. S.; Chen, Q.; Bu, X. H. Phys. Chem. Chem. Phys. 2013, 15, 5430.
[29] Yan, Z. J.; Yuan, Y.; Tian, Y. Y.; Zhang, D. M.; Zhu, G. S. Angew. Chem. Int. Ed. 2015, 54, 12733.
[30] Riley, B. J.; Chun, J.; Ryan, J. V.; Matyáš, J.; Li, X. S.; Matson, D. W.; Sundaram, S. K.; Strachan, D. M.; Vienna, J. D. RSC Adv. 2011, 1, 1704.
[31] Liu, Q. K.; Ma, J. P.; Dong, Y. B. Chem. Commun. 2011, 47, 7185.
[32] Sava, D. F.; Garino, T. J.; Nenoff, T. M. Ind. Eng. Chem. Res. 2012, 51, 614.
[33] Chen, Y. F.; Sun, H. X.; Yang, R. X.; Wang, T. T.; Pei, C. J.; Xiang, Z. T.; Zhu, Z. Q.; Liang, W. D.; Li, A.; Deng, W. Q. J. Mater. Chem. A 2015, 3, 87.
/
| 〈 |
|
〉 |