

New Advances on Nucleophilic Phosphine-Triggered Annulation Reactions of Allenoates
Received date: 2015-09-22
Online published: 2016-02-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21225208, 21532008), the National Basic Research Program of China (973 Program, No. 2014CB745100), and Tianjin Municipal Science & Technology Commission (No. 14JCZDJC33400).
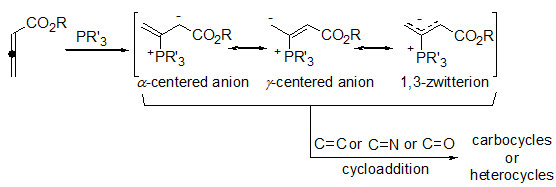
In the mid 1990s, Lu and co-workers reported the nucleophilic phosphine-triggered annulation reactions of allenoates with electron-deficient olefins or imines. As one of the most efficient and straightforward synthetic strategies for the construction of highly functionalized carbocycle or heterocycle structural motifs, the development and application of nucleophilic phosphine-triggered annulation of allenoates by using various nucleophilic phosphines and electron-deficient partners have attracted more and more interests of chemists over the past decades. Furthermore, the development of asymmetric phosphine-triggered annulation of allenoates cleaved a new way to the total synthesis of bioactive natural products and medicinally important substances. In addition, aldehydes and ketones were also employed into this reaction, leading to a wide range of O-fused heterocycles. This review focuses on the important developments concerning racemic and asymmetric annulation reactions of allenoates in the past two decades.
Yang Lijun , Ma Junan . New Advances on Nucleophilic Phosphine-Triggered Annulation Reactions of Allenoates[J]. Acta Chimica Sinica, 2016 , 74(2) : 130 -148 . DOI: 10.6023/A15090617
[1] (a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535.
(b) Valentine, D. H.; Hillhouse, J. H. Synthesis 2003, 317.
(c) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035.
(d) Lu, X.; Du, Y.; Lu, C. Pure Appl. Chem. 2005, 77, 1985.
(e) Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520.
(f) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140.
(g) Denmark, S. E.; Beutner, G. L. Angew. Chem., Int. Ed. 2008, 47, 1560.
(h) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102.
(i) Marinetti, A.; Voituriez, A. Synlett 2010, 174.
(j) Beata, K. Cent. Eur. J. Chem. 2010, 1147.
(k) Wang, S.-X.; Han, X.; Zhong, F.; Wang, Y.; Lu, Y. Synlett 2011, 2766.
(l) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 2267.
(m) Yu, S.; Ma, S. Angew. Chem., Int. Ed. 2012, 51, 3074.
(n) Xu, S.; He, Z. Chin. J. Org. Chem. 2012, 32, 1159. (徐四龙, 贺峥杰, 有机化学, 2012, 32, 1159.)
(o) Fan, Y.-C.; Kwon, O. Chem. Commun. 2013, 49, 11588.
(p) Tang, Q.; Tu, A.; Deng, Z.; Hu, M.; Zhong, W. Chin. J. Org. Chem. 2013, 33, 954. (唐谦, 涂爱平, 邓真真, 胡梦莹, 钟为慧, 有机化学, 2013, 33, 954.)
(q) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev.2014, 43, 2927.
(r) Zhou, R.; Liu, R.; Li, R.; He, Z. Chin. J. Org. Chem. 2014, 34, 2385. (周荣, 刘蓉芳, 李瑞丰, 贺峥杰, 有机化学, 2014, 34, 2385.)
(s) Xu, S.; He, Z. Chin. J. Org. Chem. 2014, 34, 2438(in Chinese). (徐四龙, 贺峥杰, 有机化学, 2014, 34, 2438.)
[2] Zhang, C.; Lu, X. J. Org. Chem. 1995, 60, 2906.
[3] (a) Xia, Y.; Liang, Y.; Chen, Y.; Wang, M.; Jiao, L.; Huang, F.; Liu, S.; Li, Y.; Yu, Z.-X. J. Am. Chem. Soc. 2007, 129, 34701.
(b) Liang, Y.; Liu, S.; Xia, Y.; Li, Y.; Yu, Z.-X. Chem. Eur. J. 2008, 14, 4361.
[4] (a) Dudding, T.; Kwon, O.; Mercier, E. Org. Lett. 2006, 8, 3643.
(b) Mercier, E.; Fonovic, B.; Henry, C.; Kwon, O.; Dudding, T. Tetrahedron Lett. 2007, 48, 3617.
[5] Xu, Z.; Lu, X. Tetrahedron Lett. 1999, 40, 549.
[6] Xu, Z.; Lu, X. Tetrahedron Lett. 1997, 38, 3461.
[7] Xu, Z.; Lu, X. J. Org. Chem. 1998, 63, 5031.
[8] Du, Y.; Lu, X.; Yu, Y. J. Org. Chem. 2002, 67, 8901.
[9] Du, Y.; Lu, X. J. Org. Chem. 2003, 68, 6463.
[10] (a) Pyne, S. G.; Schafer, K.; Skelton, B. W.; White, A. H. Chem. Commun. 1997, 2267.
(b) Ung, A. T.; Schafer, K.; Lindsay, K. B.; Pyne, S. G.; Amornraksa, K.; Wouters, R.; Van der Linden, I.; Biesmans, I.; Lesage, A. S. J.; Skelton, B. W.; White, A. H. J. Org. Chem. 2002, 67, 227.
[11] (a) Pham, T. Q.; Pyne, S. G.; Skelton, B. W.; White, A. H. J. Org. Chem. 2005, 70, 6369.
(b) Yong, S. R.; Williams, M. C.; Pyne, S. G.; Ung, A. T.; Skelton, B. W.; White, A. H.; Turner, P. Tetrahedron 2005, 61, 8120.
[12] Lu, X.; Lu, Z.; Zhang, X. Tetrahedron 2006, 62, 457.
[13] Henry, C. E.; Kwon, O. Org. Lett. 2007, 9, 3069.
[14] Lee, S. Y.; Fujiwara, Y.; Nishiguchi, A.; Kalek, M.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 4587.
[15] Guan, X.-Y.; Wei, Y.; Shi, M. Org. Lett. 2010, 12, 5024.
[16] Zou, Y.-Q.; Li, C.; Rong, J.; Yan, H.; Chen, J.-R.; Xiao, W.-J. Synlett 2011, 1000.
[17] (a) Zhang, X.-C.; Cao, S.-H.; Wei, Y.; Shi, M. Chem. Commun. 2011, 47, 1548.
(b) Zhang, X.-C.; Cao, S.-H.; Wei, Y.; Shi, M.Org. Lett. 2011, 13, 1142.
[18] Gomez, C.; Gicquel, M.; Carry, J.-C.; Schio, L.; Retailleau, P.; Voituriez, A.; Marinetti, A. J. Org. Chem. 2013, 78, 1488.
[19] (a) Shu, L.-H.; Sun, W.-Q.; Zhang, D.-W.; Wu, S.-H.; Wu, H.-M.; Xu, J.-F.; Lao, X.-F. Chem. Commun. 1997, 79.
(b) O'Donovan, B. F.; Hitchcock, P. B.; Meidine, M. F.; Kroto, H. W.; Taylor, R.; Walton, D. R. M. Chem. Commun. 1997, 81.
[20] Marco-Martinez, J.; Marcos, V.; Reboredo, S.; Filippone, S.; Martin, N. Angew. Chem., Int. Ed. 2013, 52, 5115.
[21] Kumar, K.; Kapoor, R.; Kapur, A.; Ishar, M. P. S. Org. Lett. 2000, 2, 2023.
[22] Ruano, J. L. G.; Núñez, Jr. A.; Martín, M. R.; Fraile, A. J. Org. Chem. 2008, 73, 9366.
[23] Jones, R. A.; Krische, M. J. Org. Lett. 2009, 11, 1849.
[24] Zhu, G.; Chen, Z.; Jiang, Q.; Xiao, D.; Cao, P.; Zhang, X. J. Am. Chem. Soc. 1997, 119, 3836.
[25] Wilson, J. E.; Fu, G. C. Angew. Chem., Int. Ed. 2006, 45, 1426.
[26] Fujiwara, Y.; Fu, G. C. J. Am. Chem. Soc. 2011, 133, 12293.
[27] Steurer, M.; Jensen, K. L.; Worgull, D.; Jorgensen, K. A. Chem. Eur. J. 2012, 18, 76.
[28] Cowen, B. J.; Miller, S. J. J. Am. Chem. Soc. 2007, 129, 10988.
[29] Voituriez, A.; Panossian, A.; Fleury-Bregeot, N.; Retailleau, P.; Marinetti, A. J. Am. Chem. Soc. 2008, 130, 14030.
[30] (a) Voituriez, A.; Panossian, A.; Fleury-Bregeot, N.; Retailleau, P.; Marinetti, A. Adv. Synth. Catal. 2009, 351, 1968.
(b) Neel, M.; Gouin, J.; Voituriez, A.; Marinetti, A. Synthesis 2011, 2003.
(c) Duvvuru, D.; Pinto, N.; Gomez, C.; Betzer, J.-F.; Retailleau, P.; Voituriez, A.; Marinetti, A. Adv. Synth. Catal. 2012, 354, 408.
[31] Pinto, N.; Neel, M.; Panossian, A.; Retailleau, P.; Frison, G.; Voituriez, A.; Marinetti, A. Chem. Eur. J. 2010, 16, 1033.
[32] (a) Schuler, M.; Voituriez, A.; Marinetti, A. Tetrahedron:Asymmetry 2010, 21, 1569.
(b) Voituriez, A.; Pinto, N.; Neel, M.; Retailleau, P.; Marinetti, A. Chem. Eur. J. 2010, 16, 12541.
(c) Pinto, N.; Retailleau, P.; Voituriez, A.; Marinetti, A. Chem. Commun. 2011, 47, 1015.
[33] Xiao, H.; Chai, Z.; Zheng, C.-W.; Yang, Y.-Q.; Liu, W.; Zhang, J.-K.; Zhao, G. Angew. Chem., Int. Ed. 2010, 49, 4467.
[34] Han, X.; Wang, Y.; Zhong, F.; Lu, Y. J. Am. Chem. Soc. 2011, 133, 1726.
[35] (a) Han, X.; Wang, S.-X.; Zhong, F.; Lu, Y. Synthesis 2011, 1859.
(b) Zhao, Q.; Han, X.; Wei, Y.; Shi, M.; Lu, Y. Chem. Commun. 2012, 48, 970.
[36] Zhang, X.-N.; Shi, M. ACS Catal. 2013, 3, 507.
[37] Gicquel, M.; Zhang, Y.; Aillard, P.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem., Int. Ed. 2015, 54, 5470.
[38] Tran, Y. S.; Kwon, O. J. Am. Chem. Soc. 2007, 129, 12632.
[39] Tran, Y. S.; Martin, T. J.; Kwon, O. Chem. Asian J. 2011, 6, 2101.
[40] Baskar, B.; Dakas, P.-Y.; Kumar, K. Org. Lett. 2011, 13, 1988.
[41] Li, E.; Huang, Y.; Liang, L.; Xie, P. Org. Lett. 2013, 15, 3138.
[42] Gicquel, M.; Gomez, C.; Retailleau, P.; Voituriez, A.; Marinetti, A. Org. Lett. 2013, 15, 4002.
[43] Zhong, F.; Han, X.; Wang, Y.; Lu, Y.Chem. Sci. 2012, 3, 1231.
[44] Xiao, H.; Chai, Z.; Cao, D.; Wang, H.; Chen, J.; Zhao, G. Org. Biomol. Chem. 2012, 10, 3195.
[45] Meng, W.; Zhao, H.-T.; Nie, J.; Zheng, Y.; Fu, A.; Ma, J.-A. Chem. Sci. 2012, 3, 3053.
[46] Zheng, J.; Huang, Y.; Li, Z. Org. Lett. 2013, 15, 5064.
[47] Li, E.; Huang, Y. Chem. Commun. 2014, 50, 948.
[48] (a) Li, E.; Jia, P.; Liang, L.; Huang, Y. ACS Catal. 2014, 4, 600.
(b) Li, E.; Huang, Y. Chem. Eur. J. 2014, 20, 3520.
[49] Zhu, X.-F.; Henry, C. E.; Kwon, O. Tetrahedron 2005, 61, 6276.
[50] Zhao, G.-L.; Shi, M. J. Org. Chem. 2005, 70, 9975.
[51] Tang, X.; Zhang, B.; He, Z.; Gao, R.; He, Z. Adv. Synth. Catal. 2007, 349, 2007.
[52] Zhang, B.; He, Z.; Xu, S.; Wu, G.; He, Z. Tetrahedron 2008, 64, 9471.
[53] Wang, Y.-Q.; Zhang, Y.; Dong, H.; Zhang, J.; Zhao, J. Eur. J. Org. Chem. 2013, 3764.
[54] Chen, X.-Y.; Lin, R.-C.; Ye, S. Chem. Commun. 2012, 48, 1317.
[55] (a) Yang, L.-J.; Wang, S.; Nie, J.; Li, S.; Ma, J.-A. Org. Lett. 2013, 15, 5214.
(b) Yang, L.-J.; Li, S.; Wang, S.; Nie, J.; Ma, J.-A. J. Org. Chem. 2014, 79, 3547.
[56] Jean, L.; Marinetti, A. Tetrahedron Lett. 2006, 47, 2141.
[57] Fleury-Bregeot, N.; Jean, L.; Retailleau, P.; Marinetti, A. Tetrahedron 2007, 63, 11920.
[58] Panossian, A.; Fleury-Brégeot, N.; Marinetti, A. Eur. J. Org. Chem. 2008, 3826.
[59] Pinto, N.; Fleury-Brégeot, N.; Marinetti, A. Eur. J. Org. Chem. 2009, 146.
[60] Scherer, A.; Gladysz, J. A. Tetrahedron Lett. 2006, 47, 6335.
[61] Fang, Y.-Q.; Jacobsen, E. N. J. Am. Chem. Soc. 2008, 130, 5660.
[62] Han, X.; Zhong, F.; Wang, Y.; Lu, Y. Angew. Chem., Int. Ed. 2012, 51, 767.
[63] Henry, C. E.; Xu, Q.; Fan, Y.-C.; Martin, T. J.; Belding, L.; Dudding, T.; Kwon, O. J. Am. Chem. Soc. 2014, 136, 11890.
[64] Andrews, I. P.; Kwon, O. Chem. Sci. 2012, 3, 2510.
[65] Zhu, X.-F.; Lan, J.; Kwon, O. J. Am. Chem. Soc. 2003, 125, 4716.
[66] (a) Tran, Y. S.; Kwon, O. Org. Lett. 2005, 7, 4289.
(b) Villa,R. A.; Xu,Q.; Kwon, O. Org. Lett. 2012, 14, 4634.
[67] (a) Castellano, S.; Fiji, H. D. G.; Kinderman, S. S.; Watanabe, M.; Leon, P.; Tamanoi, F.; Kwon, O. J. Am. Chem. Soc. 2007, 129, 5843.
(b) Cruz, D.; Wang, Z.; Kibbie, J.; Modlin, R.; Kwon, O. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6769.
[68] Chen, X.-Y.; Ye, S. Eur. J. Org. Chem. 2012, 5723.
[69] Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Suzuki, M.; Sasai, H. Asian J. Org. Chem. 2014, 3, 412.
[70] Wurz, R. P.; Fu,G. C. J. Am. Chem. Soc. 2005, 127, 12234.
[71] Xiao, H.; Chai, Z.; Wang, H.-F.; Wang, X.-W.; Cao, D.-D.; Liu, W.; Lu, Y.-P.; Yang, Y.-Q.; Zhao, G. Chem. Eur. J. 2011, 17, 10562.
[72] Na, R.; Jing, C.; Xu, Q.; Jiang, H.; Wu, X.; Shi, J.; Zhong, J.; Wang, M.; Benitez, D.; Tkatchouk, E.; Goddard III, W. A.; Guo, H.; Kwon, O. J. Am. Chem. Soc. 2011, 133, 13337.
[73] Wang, D.; Lei, Y.; Wei, Y.; Shi, M. Chem. Eur. J. 2014, 20, 15325.
[74] Meng, X.; Huang, Y.; Chen, R. Org. Lett. 2009, 11, 137.
[75] Meng, X.; Huang, Y.; Zhao, H.; Xie, P.; Ma, J.; Chen, R. Org. Lett. 2009, 11, 991.
[76] Sun, Y.-W.; Guan, X.-Y.; Shi, M. Org. Lett. 2010, 12, 5664.
[77] Xu, S.; Zhou, L.; Ma, R.; Song, H.; He, Z. Org. Lett. 2010, 12, 544.
[78] Xu, S.; Zhou, L.; Ma, R.; Song, H.; He, Z. Chem. Eur. J. 2009, 15, 8698.
[79] Wang, T.; Ye, S. Org. Biomol. Chem. 2011, 9, 5260.
[80] Zhu, X.-F.; Henry, C. E.; Wang, J.; Dudding, T.; Kwon, O. Org. Lett. 2005, 7, 1387.
[81] Zhu, X.-F.; Schaffner, A.-P.; Li, R. C.; Kwon, O. Org. Lett. 2005, 7, 2977.
[82] Creech, G. S.; Kwon, O. Org. Lett. 2008, 10, 429.
[83] Wang, T.; Ye, S. Org. Lett. 2010, 12, 4168.
[84] Kumar, K.; Kapur, A.; Ishar, M. P. S. Org. Lett. 2000, 2, 787.
[85] Lu, Z.; Zheng, S.; Zhang, X.; Lu, X. Org. Lett. 2008, 10, 3267.
[86] Zhang, Q.;Yang, L.; Tong, X. J. Am. Chem. Soc. 2010, 132, 2550.
[87] Gu, Y.; Hu, P.; Ni, C.; Tong, X. J. Am. Chem. Soc. 2015, 137, 6400.
[88] Han, X.; Yao, W.; Wang, T.; Tan, Y. R.; Yan, Z.; Kwiatkowski, J.; Lu, Y. Angew. Chem., Int. Ed. 2014, 53, 5643.
[89] Ziegler, D. T.; Riesgo, L.; Ikeda, T.; Fujiwara, Y.; Fu, G. C. Angew. Chem., Int. Ed. 2014, 53, 13183.
[90] Kramer, S.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 3803.
/
| 〈 |
|
〉 |