

Synthesis and Self-assembly of pH and ROS Dual Responsive Poly(β-thioester)s
Received date: 2015-12-19
Online published: 2016-02-23
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21374026, 51373046, 51473045) and Hebei Provincial Natural Science Foundation of China (B2014202209).
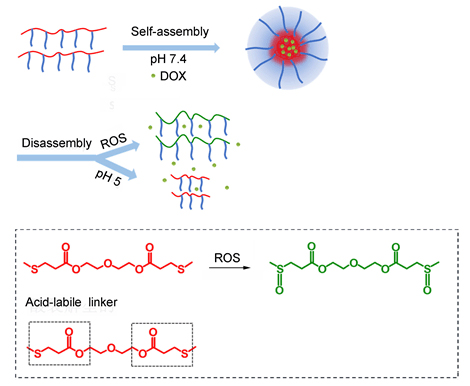
The Michael addition reaction, which is a mild reaction between activated olefins and nucleophiles, has been widely used in synthesis of tailored macromolecular architectures. We designed a copolymer nanoparticle to obtain the ROS and pH dual responsive capability. We synthesized the amphiphilic poly(β-thioester)s copolymers (D-D-P) composed of di(ethylene glycol) diacrylate (DEDA), DL-dithiothreitol (DTT), acryloyl chloride (AC) and hydrophilic PEG-SH with average Mn=2000 g·mol-1 via Michael addition reaction. The reactions are facile and controllable, and the structures of acquired copolymers are well characterized. The structures of the polymers were confirmed by 1H NMR, and the number molecular weight and distribution of the copolymer D-D-P was measured by GPC (Mn=50400). D-D-P could self-assemble into nanoparticles with core-shell structures by dialysis method due to the composition of hydrophilic side chains and hydrophobic polymer backbones. After the preparation of ROS and pH dual responsive D-D-P nanoparticles in phosphate buffer solution, the morphology and size of D-D-P nanoparticles were observed by transmission electron microscopy (TEM) and dynamic light scattering (DLS) showed that the number size distribution of nanoparticles was around 280 nm. Nile Red (NR) is a unique neutral hydrophobic molecule, which shows very weak fluorescence in aqueous solution and can emit strong fluorescence in the hydrophobic environment. NR absorption and emission spectra are strongly dependent on environment polarity, which make it as a probe molecule that is widely used in the evaluation of microenvironment polarity. The disassembly behaviors of D-D-P nanoparticles were investigated by the change of the nanoparticle size and NR fluorescence spectra. The diameter of nanoparticle decreased under pH 5 and ROS environment conditions, and the NR fluorescence also became weak under pH 5 and ROS environment conditions, which could be attributed to the gradual dissociation of nanoparticles, proving the ROS and pH dual responsive properties of the poly(β-thioester)s. The release behaviors of the DOX encapsulated D-D-P nanoparticles in acidic and oxidative condition were studied by the UV absorption and were further proved in MCF-7 cells.
Key words: poly(β-thioester)s; nanoparticles; pH-responsive; ROS-responsive; self-assembly
Zhao Wenjing , Qiao Zengying , Duan Zhongyu , Wang Hao . Synthesis and Self-assembly of pH and ROS Dual Responsive Poly(β-thioester)s[J]. Acta Chimica Sinica, 2016 , 74(3) : 234 -240 . DOI: 10.6023/A15120787
[1] Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Nano Today 2012, 7, 467.
[2] Davis, M. E.; Chen, Z. G.; Shin, D. M. Nat. Rev. Drug Discov. 2008, 7, 771.
[3] Zhu, C.; Jung, S.; Luo, S.; Meng, F.; Zhu, X.; Park, T. G.; Zhong, Z. Biomaterials 2010, 31, 2408.
[4] Stuart, M. A.; Huck, W. T.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G. B.; Szleifer, I.; Tsukruk, V. V.; Urban, M.; Winnik, F.; Zauscher, S.; Luzinov, I.; Minko, S. Nat. Mater. 2010, 9, 101.
[5] Wang, L.; Li, L. L.; Ma, H. L.; Wang, H. Chin. Chem. Lett. 2013, 24, 351.
[6] Zhang, D.; Zhao, Y.-X.; Gao, Y.-J.; Gao, F.-P.; Fan, Y.-S.; Li, X.-J.; Duan, Z.-Y.; Wang, H. J. Mater. Chem. B 2013, 1, 5100.
[7] Xiao, C.; Ding, J.; Ma, L.; Yang, C.; Zhuang, X.; Chen, X. Polym. Chem. 2015, 6, 738.
[8] Gupta, M. K.; Martin, J. R.; Werfel, T. A.; Shen, T.; Page, J. M.; Duvall, C. L. J. Am. Chem. Soc. 2014, 136, 14896.
[9] Carroll, V.; Michel, B. W.; Blecha, J.; VanBrocklin, H.; Keshari, K.; Wilson, D.; Chang, C. J. J. Am. Chem. Soc. 2014, 136, 14742.
[10] Fang, R. C.; Xu, H. P.; Cao, W.; Yang, L. L.; Zhang, X. Polym. Chem. 2015, 6, 2817.
[11] Gerweck, L. E.; Seetharaman, K. Cancer Res. 1996, 56, 1194.
[12] Tannock, I. F.; Rotin, D. Cancer Res. 1989, 49, 4373.
[13] Hu, Q.; Li, Y.; Wang, J.; Li, Y. Acta Chim. Sinica 2015, 73, 416. (胡齐,李玉祥,王静媛,李亚鹏,化学学报,2015, 73, 416.)
[14] Qi, X. J.; Liu, S. X.; Liu, T.; Dang, L.; Yang, X.; Yu, W. N.; Wang, H. M. Acta Chim. Sinica 2011, 69, 1803. (齐晓君, 刘守信, 刘腾, 党莉, 杨曦, 雨薇娜, 王红梅, 化学学报, 2011, 69, 1803.)
[15] Liu, S. X.; Zhang, C. Y.; Fang, Y.; Wang, H. X.; Chen, F. Q. Acta Chim. Sinica 2009, 67, 1910. (刘守信, 张朝阳, 房喻, 王焕霞, 陈奋强,化学学报, 2009, 67, 1910.)
[16] Qiao, Z. Y.; Zhang, D.; Hou, C. Y.; Zhao, S. M.; Liu, Y.; Gao, Y. J.; Tan, N. H.; Wang, H. J. Mater. Chem. B 2015, 3, 4514.
[17] Qiao, Z. Y.; Hou, C. Y.; Zhao, W. J.; Zhang, D.; Yang, P. P.; Wang, L.; Wang, H. Chem. Commun. 2015, 51, 12609.
[18] Qiao, Z. Y.; Hou, C. Y.; Zhang, D.; Liu, Y.; Lin, Y. X.; An, H. W.; Li, X. J.; Wang, H. J. Mater. Chem. B 2015, 3, 2943.
[19] Zaquen, N.; Wenn, B.; Ranieri, K.; Vandenbergh, J.; Junkers, T. J. Polym. Sci., Part A: Polym. Chem. 2014, 52, 178.
[20] Vandenbergh, J.; Ranieri, K.; Junkers, T. Macromol. Chem. Phys. 2012, 213, 2611.
[21] Song, C.-C.; Du, F.-S.; Li, Z.-C. J. Mater. Chem. B 2014, 2, 3413.
[22] Lallana, E.; Tirelli, N. Macromol. Chem. Phys. 2013, 214, 143.
[23] Dan, K.; Ghosh, S. Angew. Chem. Int. Ed. 2013, 52, 7300.
[24] Wu, H.; Zhu, L.; Torchilin, V. P. Biomaterials 2013, 34, 1213.
[25] Zhao, C. W.; He, P.; Xiao, C. S.; Gao, X. Y.; Zhuang, X. L.; Chen, X. S. J. Appl. Polym. Sci. 2012, 123, 2923.
[26] Yang, S. N.; Zhu, F. Y.; Wang, Q.; Liang, F. X.; Qu, X. Z.; Gan, Z. H.; Yang, Z. Z. J. Mater. Chem. B 2015, 3, 4043.
[27] Xiao, D.; Jia, H. Z.; Zhang, J.; Liu, C. W.; Zhuo, R. X.; Zhang, X. Z. Small 2014, 10, 591.
[28] Ji, X.; Chen, J.; Chi, X.; Huang, F. ACS Macro Lett. 2014, 3, 110.
[29] Sackett, D. L.; Wolff, J. Anal. Biochem. 1987, 167, 228.
[30] Liu, G. H.; Wang, X. R.; Hu, J. M.; Zhang, G. Y.; Liu, S. Y. J. Am. Chem. Soc. 2014, 136, 7492.
[31] Liu, G. H.; Zhang, G. F.; Hu, J. M.; Wang, X. R.; Zhu, M. Q.; Liu, S. Y. J. Am. Chem. Soc. 2015, 137, 11645.
/
| 〈 |
|
〉 |