

Substituent Effect on Quinoline-Malononitrile AIE Fluorescent Properties
Received date: 2016-01-03
Online published: 2016-03-03
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21325625, 21476076).
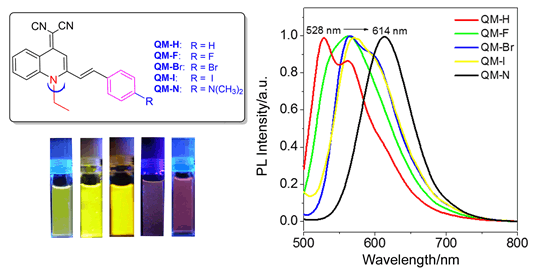
As well-known, traditional luminescent dyes such as dicyanomethylene-4H-pyran (DCM) luminogens used in biological diagnosis and therapy still exit several limitations due to their inherent molecular structures. One of the most notorious phenomena is "aggregation caused quenching" (ACQ), namely that the fluorescence can be easily observed in dilute solution, but quenched in high concentration or aggregated state. Therefore, how to understand the aggregation environment formed by dye molecules and further utilize the aggregate itself as a potential pattern for biomedical application is highly desirable. Since the intriguing discovery of the aggregation-induced emission (AIE) phenomenon, much effort has been paid to exploration of AIE systems and their applications. These AIE chromophores exhibit highly bright fluorescence when aggregated, and weak fluorescence when dissolved in solution, making them beneficial for improving the sensitivity of biosensors and bioimaging in situ or in vivo. Herein we set out to construct a novel AIE-active quinoline-malononitrile (QM) building block, by merely replacing the oxygen atom in DCM moiety with N-ethyl group, thoroughly solving the fluorescence quenching problems of DCM derivatives in aggregation. Five QM derivatives (QM-H, QM-F, QM-Br, QM-I and QM-N) with different substituent groups have been successfully synthesized by Knoevenagel reaction, extending the AIE wavelength from 528 to 614 nm in the aggregated state. A series of experiments were performed to examine the photoluminescence properties of QM-H, QM-F, QM-Br, QM-I and QM-N. As expected, all these AIE-active compounds show weak or no fluorescence in molecular state when dissolved in THF solution, but enhanced emission in solid or aggregate state along with an increasing volume fraction of water in tetrahydrofuran/water (THF/H2O) mixtures. Moreover, their AIE-active fluorescent properties are dependent upon the different aggregated microenvironment affected by substituent groups of QM derivatives. Notably, the halogen atoms of QM-F, QM-Br and QM-I play important role in AIE quantum yield, while introducing electron donor group shifts the solid fluorescence of QM-N into red emission. The substituent effect of QM derivatives with excellent AIE properties can provide a platform to develop NIR AIE materials.
Xia Zhiqing , Shao Andong , Li Qiang , Zhu Shiqin , Zhu Weihong . Substituent Effect on Quinoline-Malononitrile AIE Fluorescent Properties[J]. Acta Chimica Sinica, 2016 , 74(4) : 351 -355 . DOI: 10.6023/A16010001
[1] Guo, Z. Q.; Zhu, W. H.; Tian, H. Chem. Commun. 2012, 48, 6073.
[2] Tang, C. W.; VanSlyke, S. A.; Chen, C. H. J. Appl. Phys. 1989, 65, 3610.
[3] (a) Chen, C. H. Chem. Mater. 2004, 16, 4389.
(b) Zhong, H. L.; Lai, H.; Fang, Q. J. Phys. Chem. C 2011, 115, 2423.
[4] Luo, J. D.; Xie, Z. L.; Lam, J. W. Y.; Cheng, L.; Chen, H. Y.; Qiu, C. F.; Kwok, H. S.; Zhan, X. W.; Liu, Y. Q.; Zhu, D. B.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
[5] (a) Kwok, R. T. K.; Leung, C. W. T.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2015, 44, 4228.
(b) Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam J. W. Y.; Tang, B. Z. Chem. Rev. 2015, 115, 11718.
(c) Guo, Z. Q.; Shao, A. D.; Zhu, W. H. J. Mater. Chem. C 2016, DOI: 10. 1039/C5TC03369A.
[6] (a) Zhang, X. Q.; Zhang, X. Y.; Yang, B.; Zhang, Y. L.; Wei, Y. ACS Appl. Mater. Interfaces 2014, 6, 3600.
(b) Wang, S.; Zhu, Z.; Wei, D. Q.; Yang, C. L. J. Mater. Chem. C 2015, 3, 11902.
[7] Yao, L.; Zhang, S. T.; Wang, R.; Li, W. J.; Shen, F. Z.; Yang, B.; Ma, Y. G. Angew. Chem., Int. Ed. 2014, 53, 2119.
[8] (a) Hu, F.; Huang, Y. Y.; Zhang, G. X.; Zhao, R.; Yang, H.; Zhang, D. Q. Anal. Chem. 2014, 86, 7987.
(b) Xun, Z. Q.; Tang, H. Y.; Zeng, Y.; Chen, J. P.; Yu, T. J.; Zhang, X. H.; Li, Y. Acta Chim. Sinica 2015, 73, 819. (寻知庆, 唐海云, 曾毅, 陈金平, 于天君, 张小辉, 李嫕, 化学学报, 2015, 73, 819.)
(c) Li, Y. D.; Zhang, H.; Wang, X. C.; Wang, F.; Xia, Y. J. Acta Chim., Sinica 2015, 73, 1055. (李昱达, 张恒, 王迅昶, 汪锋, 夏养君, 化学学报, 2015, 73, 1055.)
[9] Lu, H. G.; Zheng, Y. D.; Zhao, X. W.; Wang, L. J.; Ma, S. Q.; Han, X. Q.; Xu, B.; Tian, W. J.; Gao, H. Angew. Chem. Int. Ed. 2016, 55, 155.
[10] Chi, Z. G.; Zhang, X. Q.; Xu, B. J.; Zhou, X.; Ma, C. P.; Zhang, Y.; Liu, S. W.; Xu, J. R. Chem. Soc. Rev. 2012, 41, 3878.
[11] Huang, J.; Jiang, Y. B.; Yang, J.; Tang, R. L.; Xie, N.; Li, Q. Q.; Kwok, H. S.; Tang, B. Z.; Li, Z. J. Mater. Chem. C 2014, 2, 2028.
[12] Zhang, Y. P.; Li, D. D.; Li, Y.; Yu, J. H. Chem. Sci. 2014, 5, 2710.
[13] (a) Zhang, S.; Qin, A. J.; Sun, J. Z.; Tang, B. Z. Prog. Chem. 2011, 23, 623. (张双, 秦安军, 孙景志, 唐本忠, 化学进展, 2011, 23, 623.)
(b) Zhao, G. S.; Shi, C. X.; Guo, Z. Q.; Zhu, W. H.; Zhu, S. Q. Chin. J. Org. Chem. 2012, 32, 1620. (赵国生, 史川兴, 郭志前, 朱为宏, 朱世琴, 有机化学, 2012, 32, 1620.)
(c) Liu, P.; Chen, D. D.; Feng, X.; Shi, J. B.; Tong, B.; Dong, Y. P. Imag. Sci. Photochem. 2015, 33, 441 (in Chinese). (刘派, 陈笛笛, 冯霄, 石建兵, 佟斌, 董宇平, 影像科学与光化学, 2015, 33, 441.)
(d) Yu, H. B.; Li, H. L.; Zhang, X. F.; Xiao, Y.; Fang, P. J.; Lü, C. J.; Hou, W. Acta Chim. Sinica 2015, 73, 450. (于海波, 李红玲, 张新富, 肖义, 方沛菊, 吕春娇, 侯伟, 化学学报, 2015, 73, 450.)
[14] Shi, C. X.; Guo, Z. Q.; Yan, Y. L.; Zhu, S. Q.; Xie, Y. S.; Zhao, Y. S.; Zhu, W. H.; Tian, H. ACS Appl. Mater. Interfaces 2012, 5, 192.
[15] Shao, A. D.; Guo, Z. Q.; Zhu, S. J.; Zhu, S. Q.; Shi, P.; Tian, H.; Zhu, W. H. Chem. Sci. 2014, 5, 1383.
[16] Shao, A. D.; Xie, Y. S.; Zhu, S. J.; Guo, Z. Q.; Zhu, S. Q.; Guo, J.; James, T. D.; Tian, H.; Zhu, W. H. Angew. Chem., Int. Ed. 2015, 54, 7275.
[17] Yuan, W. Z.; Yu, Z.; Lu, P.; Deng, C.; Lam, J. W. Y.; Wang, Z.; Chen, E.; Ma, Y.; Tang, B. Z. J. Mater. Chem. 2012, 22, 3323.
[18] Shen, X. Y.; Wang, Y. J.; Zhao, E. G.; Yuan, W. Z.; Liu, Y.; Lu, P.; Qin, A. J.; Ma, Y. G.; Sun, J. Z.; Tang, B. Z. J. Phys. Chem. C 2013, 117, 7334.
/
| 〈 |
|
〉 |