

Palladium-Catalyzed Allylic C—H Functionalization: The Development of New Catalytic Systems
Received date: 2016-02-03
Online published: 2016-03-18
Supported by
Project supported by the National Natural Science Foundation of China (No. 21232004).
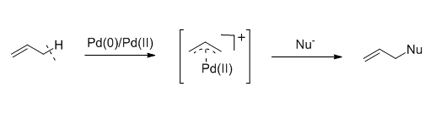
Palladium-catalyzed allylic substitution is one of the most important methodologies for the construction of C—C and C—X bonds, and has been widely applied in the synthesis of bioactive natural and pharmaceutical products. Tremendous progress has been made towards the development of increasingly elaborate nucleophiles and catalysts to facilitate the aforementioned reaction. Despite significant advances, Pd-catalyzed allylic substitution reactions remain limited to substrates possessing a good leaving group such as a carboxylate, carbonate, phosphate, or other related derivatives on the allylic moiety. Allylic alcohols and amines have also gained attention for use as substrates for Pd-catalyzed allylic substitutions, because of their use in aiding waste minimization and sustainability. Allyl groups containing allylic C—H bond(s) widely are present in numerous commercially available organic compounds and various kinds of intermediates for chemical synthesis. There is no doubt that the transformation of allylic C—H bonds into new C—C and C—X bonds is an ideal method to introduce new functional groups into molecules to construct more complex structures. However, allylic C—H functionalizations catalyzed by transition-metals are more challenging than allylic alcohols and other related allyl substrates, due to the difficult cleavage of the C—H bond and the need for a suitable oxidant. Recently, some significant advances have been reported by chemists and so Pd-catalyzed allylic C—H activations for the construction of C—C and C—X bonds have become a hot topic in the chemical community. A series of novel reactions based on new catalytic systems have been developed to produce useful molecules and complex natural products. The control of branch/linear selectivity and enantioselectivity has also been realized in the latest reports. Related work in this field is reviewed in this paper from the viewpoint of alkene substrates and nucleophiles. Pd(Ⅱ)-catalyzed asymmetric allylic C—H functionalizations are also introduced. The advantages and disadvantages of different kinds of catalytic systems (including DMSO, bissulfoxide, PPh3 and phosphoramidate as ligands) are discussed. Finally, pathways for future developments have been proposed.
Key words: C—H functionalization; allylic; coupling reaction; palladium-catalyzed; atom economy
Tang Haoming , Huo Xiaohong , Meng Qinghua , Zhang Wanbin . Palladium-Catalyzed Allylic C—H Functionalization: The Development of New Catalytic Systems[J]. Acta Chimica Sinica, 2016 , 74(3) : 219 -233 . DOI: 10.6023/A16020078
[1] (a) Godleski, S. A. In Comprehensive Organic Synthesis, Eds.: Trost, B. M.; Fleming, I., Pergamon Press, New York, 1991, p. 585.
(b) Tsuji, J. Transition Metal Reagents and Catalysts, Wiley, New York, 2000.
(c) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
(d) Trost, B. M.; Machacek, M. R.; Aponick, A. Acc. Chem. Res. 2006, 39, 747.
(e) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258.
[2] For selected reviews:
(a) Sundararaju, B.; Achard, M.; Bruneau, C. Chem. Soc. Rev. 2012, 41, 4467.
(b) Nicholas, B.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
(c) Zhang, X.; Sun, X.; Tan, J.; Fan, H.; Rao, W. Chin. J. Org. Chem. 2015, 35, 2049 (in Chinese). (张小祥, 孙小萍, 谈继淮, 樊辉, 饶卫东, 有机化学, 2015, 35, 2049.)
[3] For selected recent papers:
(a) Ozawa, F.; Okamoto, H.; Kawagishi, K.; Yamamoto, S.; Minami, T.; Yoshifuji, M. J. Am. Chem. Soc. 2002, 124, 10968.
(b) Jiang, G.; List, B. Angew. Chem., Int. Ed. 2011, 50, 9471.
(c) Zhao, X.; Liu, D.; Guo, H.; Liu, Y.; Zhang, W. J. Am. Chem. Soc. 2011, 133, 19354.
(d) Wu, X.-S.; Chen, Y.; Li, M.-B.; Zhou, M.-G.; Tian, S.-K. J. Am. Chem. Soc. 2012, 134, 14694.
(e) Li, M.-B.; Wang, Y.; Tian, S.-K. Angew. Chem., Int. Ed. 2012, 51, 2968.
(f) Tao, Z.-L.; Zhang, W.-Q.; Chen, D.-F.; Adele, A. Gong, L.-Z. J. Am. Chem. Soc. 2013, 135, 9255.
(g) Huo, X.; Yang, G.; Liu, D.; Liu, Y.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2014, 53, 6776.
(h) Banerjee, D.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2014, 53, 13049.
(i) Huo, X.; Quan, M.; Yang, G.; Zhao, X.; Liu, D.; Liu, Y.; Zhang, W. Org. Lett. 2014, 16, 1570.
(j) Wu, X.; Lin, H.-C.; Li, M.-L.; Li, L.-L.; Han. Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2015, 137, 13476.
[4] For selected recent reviews:
(a) Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W. Chem. Rev. 2015, 115, 1622.
(b) Yang, L.; Huang, H. Chem. Rev. 2015, 115, 3468.
(c) Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, X.; Shi, R.; Lei, A. Chem. Rev. 2015, 115, 12138.
(d) Yu, J.-Q.; Ding, K. Acta Chim. Sinica 2015, 73, 1223 (in Chinese). (余金权, 丁奎岭, 化学学报, 2015, 73, 1223.)
(e) Zhou, L.; Lu, W. Acta Chim. Sinica 2015, 73, 1250 (in Chinese). (周励宏, 陆文军, 化学学报, 2015, 73, 1250.)
(f) Shang, X.; Liu, Z. Acta Chim. Sinica 2015, 73, 1275 (in Chinese). (尚筱洁, 柳忠全, 化学学报, 2015, 73, 1275.)
(g) Liao, G.; Shi, B.-F. Acta Chim. Sinica 2015, 73, 1283 (in Chinese). (廖港, 史炳锋, 化学学报, 2015, 73, 1283.)
(h) Yang, G.; Nicholas, B.; Zhang, W. Chin. J. Catal. 2016, 37, 98. For selected recent papers:
(i) Wang, G.-W.; Zhou, A.-X.; Li, S.-X.; Yang, S.-D. Org. Lett. 2014, 16, 3118.
(j) Zhang, H.; Hu, R.-B.; Liu, N.; Li, S.-X.; Yang, S.-D. Org. Lett. 2016, 18, 28.
[5] Parshall, G.; Wilkinson, G. Inorg. Chem. 1962, 1, 896.
[6] Trost, B. M.; Fullerton, T. J. Am. Chem. Soc. 1973, 95, 292.
[7] Beccalli, E.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318.
[8] (a) Heumann, A.; Reglier, M.; Waegell, B. Angew. Chem., Int. Ed. Eng. 1982, 21, 366.
(b) Heumann, A.; Kermark, B. Angew. Chem., Int. Ed. Engl. 1984, 23, 453.
(c) McMurry, J.; Kocovsky, P. Tetrahedron Lett. 1984, 25, 4187.
[9] Franzén, J.; Backväll, J.-E. J. Am. Chem. Soc. 2003, 125, 6056.
[10] Piera, J.; Närhi, K.; Backväll, J.-E. Angew. Chem., Int. Ed. 2006, 45, 6914.
[11] Chen, M. S.; White, M. C. J. Am. Chem. Soc. 2004, 126, 1346.
[12] Fraunhoffer, K. J.; Prabagaran, N.; Sirois, L. E.; White, M. C. J. Am. Chem. Soc. 2006, 128, 9032.
[13] Covell, D. J.; Vermeulen, N. A.; Laben, N. A.; White, M. C. Angew. Chem., Int. Ed. 2006, 45, 8217.
[14] Gormisky, P.; White, M. C. J. Am. Chem. Soc. 2011, 133, 12584.
[15] Osberger, T. J.; White, M. C. J. Am. Chem. Soc. 2014, 136, 11176.
[16] Ammann, S. E.; Rice, G. T.; White, M. J. Am. Chem. Soc. 2014, 136, 10834.
[17] Fraunhoffer, K. J.; White, M. C. J. Am. Chem. Soc. 2007, 129, 7274.
[18] Wu, L.; Qiu, S.; Liu, G. Org. Lett. 2009, 11, 2707.
[19] Rice, G. T.; White, M. C. J. Am. Chem. Soc. 2009, 131, 11707.
[20] Strambeanu, I. I.; White, M. C. J. Am. Chem. Soc. 2013, 135, 12032.
[21] Liu, G.; Yin, G.; Wu, L. Angew. Chem., Int. Ed. 2008, 47, 4733.
[22] Reed, S. A.; White, M. C. J. Am. Chem. Soc. 2008, 130, 3316.
[23] (a) Du, H.; Yuan, W.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2007, 129, 7496.
(b) Du, H.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2008, 130, 8590.
[24] Lin, S.; Song, C.-X.; Cai, G.-X.; Wang, W.-H.; Shi, Z.-J. J. Am. Chem. Soc. 2008, 130, 12901.
[25] Young, A. J.; White, M. C. J. Am. Chem. Soc. 2008, 130, 14090.
[26] Young, A. J.; White, M. C. Angew. Chem., Int. Ed. 2011, 50, 6824.
[27] Howell, J. M.; Liu, W.; Young, A. J.; White, M. C. J. Am. Chem. Soc. 2014, 136, 5750.
[28] Trost, B. M.; Hansmann, M. M.; Thaisrivongs, D. A. Angew. Chem., Int. Ed. 2012, 51, 4950.
[29] Trost, B. M.; Thaisrivongs, D. A.; Hansmann, M. M. Angew. Chem., Int. Ed. 2012, 51, 11522.
[30] Covell, D. J.; White, M. C. Angew. Chem., Int. Ed. 2008, 47, 6448.
[31] Wang, P.-S.; Liu, P.; Zhai, Y.-J.; Lin, H.-C.; Han, Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2015, 137, 12732.
[32] Trost, B. M.; Thaisrivongs, D. A.; Donckele, E. J. Angew. Chem., Int. Ed. 2013, 52, 1523.
[33] Tang, S.; Wu, X.; Liao, W.; Liu, K.; Liu, C.; Luo, S.; Lei, A. Org. Lett. 2014, 16, 3584.
[34] Wang, P.-S.; Lin, H.-C.; Zhai, Y.-J.; Han, Z.-Y.; Gong, L.-Z. Angew. Chem., Int. Ed. 2014, 53, 12218.
/
| 〈 |
|
〉 |