

Synthesis of a Linear-Hyperbranched Supramolecular Polymer and Its Light-Responsive Self-Assembly Behavior
Received date: 2016-02-01
Online published: 2016-03-22
Supported by
Project supported by the National Basic Research Program (No. 2013CB834506), the China National Funds for Distinguished Young Scholar (No. 21225420), the National Natural Science Foundation of China (Nos. 21474062, 91527304).
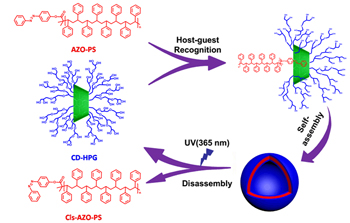
Herein we reported the synthesis, self-assembly and light-responsive disassembly of a “linear-hyperbranched” supramolecular polymer. Firstly, a hyperbranched polymer CD-g-HPG composed of hyperbranched polyglycerol with a β-cyclodextrin group in the center was synthesized by ring-opening multibranching polymerization (ROMBP). Secondly, a linear polymer AZO-PS composed of polystyrene with an azobenzene group at the end was synthesized via atom transfer radical polymerization (ATRP). Then, the linear AZO-PS and hyperbranched CD-g-HPG were conjugated together through the specific CD/AZO host-guest interactions, leading to the formation of the “linear-hyperbranched” supramolecular polymer PS-b-HPG. This supramolecular polymer was amphiphilic and could self-assemble into vesicles in water. The host-guest complexation ability was characterized by UV-Vis titration. In the case of keeping the concentration of AZO-PSs unchanged, the absorption peak at 330 nm increased gradually with the addition of CD-g-HPGs, which supported the occurrence of complexation between β-CD groups in CD-g-HPGs and AZO groups in AZO-PSs. The host-guest CD/AZO complexation constant of 4.14×104 M-1 was calculated by the Benesi-Hildebrand plot. A Job plot was generated, from which it was determined that the binding stoichiometry between AZO-PS and CD-g-HPG is 1:1. The self-assemblies of the amphiphlic linear-hyperbranched supramolecular polymers were characterized by SEM and TEM. The SEM images showed that the self-assemblies were spherical particles, and the holes directly be seen in some particles indicated that they were vesicles or hollow spheres with a very thin wall thickness. The TEM images of self-assemblies stained with ruthenium tetroxide (RuO4) indicated that the spherical particles were vesicles according to a clear contrast difference between the inner pool and the outer thin wall. At last, we showed that the vesicles could disassemble under UV light due to the trans-to-cis isomerisation of the AZO groups. With the continuous UV irradiation on vesicles, the absorption peak of trans-AZO diminished gradually and almost completely disappeared after 900 seconds. Meanwhile, the solution was transformed from turbid to transparent followed with the appearance of yellow precipitates in the bottom of the bottle.
Li Huimei , Wang Jie , Ni Yunzhou , Zhou Yongfeng , Yan Deyue . Synthesis of a Linear-Hyperbranched Supramolecular Polymer and Its Light-Responsive Self-Assembly Behavior[J]. Acta Chimica Sinica, 2016 , 74(5) : 415 -421 . DOI: 10.6023/A16020076
[1] Yang, S. K.; Ambade, A. V.; Weck, M. Chem. Soc. Rev. 2011, 40, 129.
[2] Brunsveld, L.; Folmer, B. J. B.; Meijer, E. W.; Sijbesma, R. P. Chem. Rev. 2001, 101, 4071.
[3] Aida, T.; Meijer, E. W.; Stupp, S. I. Science 2012, 335, 813.
[4] Appel, E. A.; del Barrio, J.; Loh, X. J.; Scherman, O. A. Chem. Soc. Rev. 2012, 41, 6195.
[5] Hofmeier, H.; Schubert, U. S. Chem. Soc. Rev. 2004, 33, 373.
[6] Chen, G.-S.; Jiang, M. Chem. Soc. Rev. 2011, 40, 2254.
[7] Yan, X.-Z.; Wang, F.; Zheng, B.; Huang, F.-H. Chem. Soc. Rev. 2012, 41, 6042.
[8] Wang, Q.; Cheng, M.; Cao, Y.-H.; Jiang, J.-L.; Wang, L.-Y. Acta Chim. Sinica 2016, 74, 9. (王其, 程明, 曹逸涵, 强琚莉, 王乐勇, 化学学报, 2016, 74, 9.)
[9] Yi, J.-M.; Xiao, X.; Zhang, Y.-Q.; Xue, S.-F.; Tao, Z.; Zhang, J.-X. Acta Chim. Sinica 2014, 72, 949. (易君明, 肖欣, 张云黔, 薛赛凤, 陶朱, 张建新, 化学学报, 2014, 72, 949.)
[10] Zhang, M.-M.; Xu, D.-H.; Yan, X.-Z.; Chen, J.-Z.; Dong, S.-Y.; Zheng, B.; Huang, F.-H. Angew. Chem., Int. Ed. 2012, 51, 7011.
[11] Yan, X.-Z.; Xu, D.-H.; Chi, X.-D.; Chen, J.-Z.; Dong, S.-Y.; Ding, X.; Yu, Y.-H.; Huang, F.-H. Adv. Mater. 2012, 24, 362.
[12] Wang, D.-L.; Chen, H.-Y.; Su, Y.; Qiu, F.; Zhu, L.-J.; Huan, X.-Y.; Zhu, B.-S.; Yan, D.-Y.; Guo, F.-L.; Zhu, X.-Y. Polym. Chem. 2013, 4, 85.
[13] Zheng, B.; Wang, F.; Dong, S.-Y.; Huang, F.-H. Chem. Soc. Rev. 2012, 41, 1621.
[14] Zhang, H.-T.; Fan, X.-D.; Suo, R.-T.; Li, H.; Yang, Z.; Zhang, W.-B.; Bai, Y.; Yao, H.; Tian, W. Chem. Commun. 2015, 51, 15366.
[15] Tao, W.; Liu, Y.; Jiang, B.-B.; Yu, S.-R.; Huang, W.; Zhou, Y.-F.; Yan, D.-Y. J. Am. Chem. Soc. 2012, 134, 762.
[16] Liu, Y.; Yu, C.-Y.; Jin, H.-B.; Jiang, B.-B.; Zhu, X.-Y.; Zhou, Y.-F.; Lu, Z.-Y.; Yan D.-Y. J. Am. Chem. Soc. 2013, 135, 4765.
[17] Dong, R.-J.; Zhou, Y.-F.; Zhu, X.-Y. Acc. Chem. Res. 2014, 47, 2006.
[18] Wurm, F.; Frey, H. Prog. Polym. Sci. 2011, 36, 1.
[19] Kricheldorf, H. R.; Stukenbrock, T. J. Polym. Sci., Part A: Polym. Chem. 1998, 36, 31.
[20] Istratov, V.; Kautz, H.; Kim, Y. K.; Schubert, R.; Frey, H. Tetrahedron 2003, 59, 4017.
[21] Barriau, E.; Marcos, A. G.; Kautz, H.; Frey, H. Macromol. Rapid Commun. 2005, 26, 862.
[22] Wurm, F.; Nieberle, J.; Frey, H. Macromolecules 2008, 41, 1184.
[23] Jiang, W.-F.; Chen, J.-X.; Yu, S.-R.; Zhou, Y.-F.; Yan, D.-Y. Acta Polymerica Sinica 2014, (10), 1398. (江文峰, 陈建新, 吁松瑞, 周永丰, 颜德岳, 高分子学报, 2014, (10), 1398.)
[24] Chou, T. M.; Prayoonthong, P.; Aitouchen, A.; Libera, M. Polymer. 2002, 43, 2085.
[25] Zhang, D. P.; Fan, Y. J.; Li, H. M.; Li, K.; Yao, Y.; Zhou, Y. F.; Yan, D. Y. RSC Adv. 2015, 5, 47762.
[26] Crupi, V.; Ficarra, R.; Guardo, M.; Majolino, D.; Stancanelli, R.; Venuti, V. J. Pharm. Biomed. Anal. 2007, 44, 110.
[27] Rekharsky, M. V.; Inoue, Y. Chem. Rev. 1998, 98, 1875.
[28] Dong, R.-J.; Zhu, B.-S.; Zhou, Y.-F.; Yan, D.-Y.; Zhu, X.-Y. Polym. Chem. 2013, 4, 912.
[29] Zhang, D.-P. M.S. Thesis, Shanghai Jiao Tong University, Shanghai, 2015. (张大朋, 硕士论文, 上海交通大学, 上海, 2015.)
/
| 〈 |
|
〉 |