

Nitrogen Group Retaining Reaction in the Transformation of Diazo Compounds
Received date: 2016-03-29
Online published: 2016-04-26
Supported by
Project supported by the scientific research funding of Tianjin Normal University (No. 5RL138).
Diazo compounds represent a type of very important synthetic intermediates, which demonstrate wide applications in organic synthesis, continuous-in-flow technology, polymer synthesis, medicinal chemistry, chemical biology, material science and many other fields. On the other hand, diazo intermediates can be easily prepared from commercial available substrates through facile transformations, such as base-promoted decomposition of N-tosylhydrazones, diazo-transfer reaction, diazotization of alkyl amines, oxidation of hydrazones, decomposition of N-nitroso compounds. Traditional transformations of diazo compounds include nucleophilic addition/substitution by using diazo compounds as the nucleophiles, ylide type reactions, dimerization or olefination, Wolff rearrangement, transition-metal-carbene or carbenoid mediated X—H insertion reactions, catalytic cyclopropanations or cyclopropenations, and the recently developed transition-metal-catalyzed carbenoid cross-coupling reactions. In addition to these classic reactions, the diazo compounds also undergo nitrogen group retaining reactions, in which the diazo moiety is incorporated into the nitrogen-containing moiety in the target molecules. This strategy has provided an efficient and selective synthetic approach towards nitrogen atom containing functional molecules, especially for the synthesis of various N-heterocyclic compounds. Among them, the enantioselective C—N bond forming reaction as well as the asymmetric N-heterocyclic scaffold construction has important synthetic value and remains great challenge to the organic chemists. Thus, nitrogen component retaining reactions of diazo compounds has opened up a superior avenue in organic synthesis. Considering about the significant importance and the great growth in the past decade of this area, this review article will focus on the nitrogen group retaining reaction of diazo compounds. According to the reaction mechanism of these transformations, this review will be divided into the following parts: diazo compounds as nucleophiles, diazo compounds as 1,3-dipoles in cycloaddition reaction, diazo compounds as electrophiles, intramolecular reactions of vinyldiazo compounds, reduction reaction, and miscellaneous transformation. We hope that this review will corroborate the practical use of this research area as a convenient and valuable synthetic strategy.
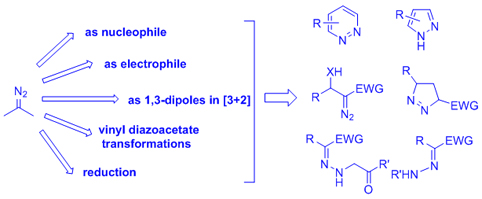
Key words: diazo compound; organic synthesis; N-heterocycle; cycloaddition
Qiu Di , Qiu Menglong , Ma Rong , Zhang Yan , Wang Jianbo . Nitrogen Group Retaining Reaction in the Transformation of Diazo Compounds[J]. Acta Chimica Sinica, 2016 , 74(6) : 472 -487 . DOI: 10.6023/A16030153
[1] (a) Ye, T.; McKervey, M. A. Chem. Rev. 1994, 94, 1091;
(b) Zhang, Z.; Wang, J. Tetrahedron 2008, 64, 6577;
(c) Padwa, A.; Austin, D. J. Angew. Chem. Int. Ed. Engl. 1994, 33, 1797;
(d) Padwa, A.; Weingarten, M. D. Chem. Rev. 1996, 96, 223;
(e) Doyle, M. P.; Forbes, D. C. Chem. Rev. 1998, 98, 911;
(f) Padwa, A. J. Organomet. Chem. 2001, 617-618, 3;
(g) Davies, H. M. L.; Antoulinakis, E. G. J. Organomet. Chem. 2001, 617-618, 47.
(h) Timmons, D. J.; Doyle, M. P. J. Organomet. Chem. 2001, 617-618, 98.
(i) Hodgson, D. M.; Pierard, F. Y. T. M.; Stupple, P. A. Chem. Soc. Rev. 2001, 30, 50;
(j) Davies, H. M. L.; Beckwith, R. E. J. Chem. Rev. 2003, 103, 2861.
(k) Singh, G. S.; Mdee, L. K. Curr. Org. Chem. 2003, 7, 1821.
(l) Gois, P. M. P.; Afonso, C. A. M. Eur. J. Org. Chem. 2004, 3773.
(m) Díaz-Requejo, M. M.; Pérez, P. J. J. Organomet. Chem. 2005, 690, 5441;
(n) Fulton, J. R.; Aggarwal, V. K.; de Vicente, J. Eur. J. Org. Chem. 2005, 1479;
(o) Davies, H. M. L.; Nikolai, J. Org. Biomol. Chem. 2005, 3, 4176.
(p) Singh, G. S. Curr. Org. Synth. 2005, 2, 377.
(q) Wee, A. G. H. Curr. Org. Synth. 2006, 3, 499.
(r) Noels, A. F. Angew. Chem. Int. Ed. 2007, 46, 1208.
[2] (a) Doyle, M. P.; McKervey, M. A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley-Interscience: New York, 1998.
(b) For the recent comprehensive review of diazo compounds, see: Ford, A.; Miel, H.; Ring, A.; Slattery, C. N.; Maguire, A. R.; McKervey, M. A. Chem. Rev. 2015, 115, 9981.
[3] Maas, G. Angew. Chem. Int. Ed. 2009, 48, 8186.
[4] For typical research recently developed by Chinese chemists, see: (a) Cheng, Q.; Xu, H.; Zhu, S.; Zhou, Q. Acta Chim. Sinica 2015, 73, 326 (in Chinese).(程清卿, 许唤, 朱守非, 周其林, 化学学报, 2015, 73, 326.)
(b) Tang, M.; Wu, Y.; Liu, Y.; Cai, M.; Xia, F.; Liu, S.; Hu, W. Acta Chim. Sinica 2016, 74, 54 (in Chinese).(唐敏, 吴永, 刘源, 蔡茂强, 夏飞, 刘顺英, 胡文浩, 化学学报, 2016, 74, 54.)
(c) Li, Y.; Huang, Z.; Xu, P.-F.; Zhang, Y.; Wang, J. Acta Chim. Sinica 2012, 70, 2024 (in Chinese).(李玉叶, 黄重行, 许鹏飞, 张艳, 王剑波, 化学学报, 2012, 70, 2024.)
[5] Zhang, Y.; Wang, J. Chem. Commun. 2009, 5350.
[6] Hashimoto, T.; Maruoka, K. Bull. Chem. Soc. Jpn. 2013, 86, 1217.
[7] Yao, W.; Wang, J. Org. Lett. 2003, 5, 1527.
[8] (a) Arai, S.; Hasegawa, K.; Nishida, A. Tetrahedron Lett. 2004, 45, 1023.
(b) Hasegawa, K.; Arai, S.; Nishida, A. Tetrahedron 2006, 62, 1390.
[9] (a) Likhar, P. R.; Roy, S.; Roy, M.; Subhas, M. S.; Kantam, M. L. Synlett 2008, 1283.
(b) Likhar, P. R.; Roy, S.; Roy, M.; Subhas, M. S.; Kantam, M. L. Catal. Commun. 2009, 10, 728.
[10] Trost, B. M.; Malhotra, S.; Koschker, P.; Ellerbrock, P. J. Am. Chem. Soc. 2012, 134, 2075.
[11] Uraguchi, D.; Sorimachi, K.; Terada, M. J. Am. Chem. Soc. 2005, 127, 9360.
[12] Hashimoto, T. ; Maruoka, K. J. Am. Chem. Soc. 2007, 129, 10054.
[13] Hashimoto, T.; Maruoka, K. Synthesis 2008, 3703.
[14] Hashimoto, T.; Kimura, H.; Nakatsu, H.; Maruoka, K. J. Org. Chem. 2011, 76, 6030.
[15] Doyle, M. P.; Kundu, K.; Russell, A. E. Org. Lett. 2005, 7, 5171.
[16] Kundu, K.; Doyle, M. P. Tetrahedron: Asymmetry 2006, 17, 574.
[17] Liu, Y.; Zhang, Y.; Jee, N.; Doyle, M. P. Org. Lett. 2008, 10, 1605.
[18] Zhou, L.; Doyle, M. P. Org. Lett. 2010, 12, 796.
[19] Xu, X.; Hu, W.-H.; Doyle, M. P. Angew. Chem. Int. Ed. 2011, 50, 6392.
[20] Mao, H.; Lin, A.; Shi, Y.; Mao, Z.; Zhu, X.; Li, W.; Hu, H.; Cheng, Y.; Zhu, C. Angew. Chem. Int. Ed. 2013, 52, 6288.
[21] Peng, C.; Cheng, J.; Wang, J. J. Am. Chem. Soc. 2007, 129, 8708.
[22] Ye, F.; Wang, C.; Zhang, Y.; Wang, J. Angew. Chem. Int. Ed. 2014, 53, 11625.
[23] (a) Rodriguez, J. B. Synthesis 2014, 46, 1129.
(b) Dubrovskiy, A. V.; Markina, N. A.; Larock, R. C. Org. Biomol. Chem. 2013, 11, 191.
(c) Hashimoto, T.; Maruoka, K. Org. Biomol. Chem. 2008, 6, 829.
[24] (a) Lozhkin, S. S.; Petrov, D. V.; Dokichev, V. A.; Tomilov, Y. V.; Nefedov, O. M. Chem. Heterocycl. Comp. 2009, 45, 937.
(b) Novikov, R. A.; Platonov, D. N.; Dokichev, V. A.; Tomilov, Y. V.; Nefedov, O. M. Russ. Chem. Bull. Int. Ed. 2010, 59, 984.
(c) Ovchinnikov, M. Y.; Yangirov, T. A.; Lobov, A. N.; Sultanova, R. M.; Khursan, S. L. Int. J. Chem. Kinet. 2013, 45, 499.
(d) Krishna, P. R.; Sekhar, E. R.; Mongin, F. Tetrahedron Lett. 2008, 49, 6768;
(e) Sun, H.; Wang, X.; Zhan, M.; Liu, J.; Xie, Y. Tetrahedron Lett. 2013, 54, 3846.
(f) Wang, W.; Simovic, D. D.; Di, M.; Fieber, L.; Rein, K. S. Bioorg. Med. Chem. Lett. 2013, 23, 1949.
(g) Ruano, J. L. G.; Alonso, M.; Cruz, D.; Fraile, A.; Martín, M. R.; Peromingo, M. T.; Tito, A.; Yuste, F. Tetrahedron 2008, 64, 10546.
(h) Cruz, D. C.; Yuste, F.; Martín, M. R.; Tito, A.; Ruano, J. L. G. J. Org. Chem. 2009, 74, 3820.
(i) Kissane, M.; Lawrence, S. E.; Maguire, A. R. Org. Biomol. Chem. 2010, 8, 2735.
(j) Hamadi, N. B.; Msaddek, M. C. R. Chimie 2011, 14, 997.
(k) Goulioukina, N. S.; Makukhin, N. N.; Beletskaya, I. P. Tetrahedron 2011, 67, 9535;
(l) Liu, R.; Yin, J.; Li, J.; Wu, J.; Chen, G.; Jin, Y.; Wang, J. Chin. J. Org. Chem. 2012, 32, 544 (in Chinese).(刘冉冉, 殷军港, 李家柱, 武进, 陈冠龙, 金英学, 王进军, 有机化学, 2012, 32, 544.)
(m) Yang, Z.; Wang, Z.; Xu, X.; Liu, Y.; Qi, C.; Wang, J. Chin. J. Org. Chem. 2012, 32, 2099 (in Chinese).(杨泽, 王振, 徐希森, 刘洋, 祁彩霞, 王进军, 有机化学, 2012, 32, 2099.)
(n) Xie, J.-W.; Wang, Z.; Yang, W.-J.; Kong, L.-C.; Xu, D.-C. Org. Biomol. Chem. 2009, 7, 4352;
(o) Maurer, S.; Jikyo, T.; Maas, G. Eur. J. Org. Chem. 2009, 2195;
(p) Hou, Y.; Cai, C.; Yu, G. Synlett 2016, 27, DOI: 10.1055/s-0035-1560596.
[25] (a) Gioiello, A.; Khamidullina, A.; Fulco, M. C.; Venturoni, F.; Zlotsky, S.; Pellicciari, R. Tetrahedron Lett. 2009, 50, 5978;
(b) Wang, L.; Huang, J.; Gong, X.; Wang, J. Chem. Eur. J. 2013, 19, 7555.
[26] (a) Slobodyanyuk, E. Y.; Artamonov, O. S.; Shishkin, O. V.; Mykhailiuk, P. K. Eur. J. Org. Chem. 2014, 2487;
(b) Mykhailiuk, P. K. Chem. Eur. J. 2014, 20, 4942;
(c) Artamonov, O. S.; Mykhailiuk, P. K.; Voievoda, N. M.; Volochnyuk, D. M.; Komarov, I. V. Synthesis 2010, 443;
(d) Artamonov, O. S.; Slobodyanyuk, E. Y.; Shishkin, O. V.; Komarov, I. V.; Mykhailiuk, P. K. Synthesis 2013, 45, 225;
(e) Li, T.-R.; Duan, S.-W.; Ding, W.; Liu, Y.-Y.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. J. Org. Chem. 2014, 79, 2296.
[27] (a) Muruganantham, R.; Namboothiri, I. J. Org. Chem. 2010, 75, 2197;
(b) Verma, D.; Mobin, S.; Namboothiri, I. N. N. J. Org. Chem. 2011, 76, 4764;
(c) Kumar, R.; Namboothiri, I. N. N. Org. Lett. 2011, 13, 4016;
(d) Kumar, R.; Nair, D.; Namboothiri, I. N. N. Tetrahedron 2014, 70, 1794;
(e) Shelke, A. M.; Suryavanshi, G. Org. Biomol. Chem. 2015, 13, 8669.
[28] (a) Suga, H.; Furihata, Y.; Sakamoto, A.; Itoh, K.; Okumura, Y.; Tsuchida, T.; Kakehi, A.; Baba, T. J. Org. Chem. 2011, 76, 7377;
(b) Gao, L.; Hwang, G.-S.; Lee, M. Y.; Ryu, D. H. Chem. Commun. 2009, 5460;
(c) Lee, S. I.; Kim, K. E.; Hwang, G.-S.; Ryu, D. H. Org. Biomol. Chem. 2015, 13, 2745;
(d) Du, T.; Du, F.; Ning, Y.; Peng, Y. Org. Lett. 2015, 17, 1308.
[29] (a) Mohanan, K.; Martin, A. R.; Toupet, L.; Smietana, M.; Vasseur, J.-J. Angew. Chem. Int. Ed. 2010, 49, 3196;
(b) Martin, A. R.; Mohanan, K.; Toupet, L.; Vasseur, J.-J.; Smietana, M. Eur. J. Org. Chem. 2011, 3184.
[30] (a) He, S.; Chen, L.; Niu, Y.-N.; Wu, L.-Y.; Liang, Y.-M. Tetrahedron Lett. 2009, 50, 2443;
(b) Cheung, K. M. J.; Reynisson, J.; McDonald, E. Tetrahedron Lett. 2010, 51, 5915;
(c) McGrath, N. A.; Raines, R. T. Chem. Sci. 2012, 3, 3237.
(d) Pramanik, M. M. D.; Kant, R.; Rastogi, N. Tetrahedron 2014, 70, 5214;
(e) Vuluga, D.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Green Chem. 2009, 11, 156;
(f) Friscourt, F.; Fahrni, C. J.; Boons, G.-J. Chem. Eur. J. 2015, 21, 13996.
[31] Li, F.; Nie, J.; Sun, L.; Zheng, Y.; Ma, J.-A. Angew. Chem. Int. Ed. 2013, 52, 6255.
[32] (a) Mykhailiuk, P. K. Angew. Chem. Int. Ed. 2015, 54, 6558;
(b) Mykhailiuk, P. K. Org. Biomol. Chem. 2015, 13, 3438;
(c) Mykhailiuk, P. K. Eur. J. Org. Chem. 2015, 7235.
[33] (a) Shoji, Y.; Hari, Y.; Aoyama, T. Tetrahedron Lett. 2004, 45, 1769;
(b) Jin, T.; Yamamoto, Y. Angew. Chem. Int. Ed. 2007, 46, 3387;
(c) Liu, Z.; Shi, F.; Martinez, P. D. G.; Raminelli, C.; Larock, R. C. J. Org. Chem. 2008, 73, 219;.
(d) Hari, Y.; Sone, R.; Aoyama, T. Org. Biomol. Chem. 2009, 7, 2804;
(e) Wang, C.-D.; Liu, R.-S. Org. Biomol. Chem. 2012, 10, 8948;
(f) Li, P.; Zhao, J.; Wu, C.; Larock, R. C.; Shi, F. Org. Lett. 2011, 13, 3340;
(g) Li, P.; Wu, C.; Zhao, J.; Rogness, D. C.; Shi, F. J. Org. Chem. 2012, 77, 3149.
[34] (a) Pérez-Aguilar, M. C.; Valdés, C. Angew. Chem. Int. Ed. 2013, 52, 7219;
(b) Sha, Q.; Wei, Y. Synthesis 2013, 45, 413;
(c) Merchant, R. R.; Allwood, D. M.; Blakemore, D. C.; Ley, S. V. J. Org. Chem. 2014, 79, 8800;
(d) Pérez-Aguilar, M. C.; Valdés, C. Angew. Chem. Int. Ed. 2015, 54, 13729.
[35] (a) Kang, T.; Kim, W.-Y.; Yoon, Y.; Kim, B. G.; Lee, H.-Y. J. Am. Chem. Soc. 2011, 133, 18050;
(b) Qiao, Y.; Han, K.-L. Org. Biomol. Chem. 2014, 12, 1220;
(c) Lee, H.-Y. Acc. Chem. Res. 2015, 48, 2308.
[36] Zhang, F.-G.; Wei, Y.; Yi, Y.-P.; Nie, J.; Ma, J.-A. Org. Lett. 2014, 16, 3122.
[37] (a) Dullweber, F.; Montforts, F.-P. Synlett 2008, 3213;
(b) Mlostoń, G.; Urbaniak, K.; Linden, A.; Heimgartner, H. Tetrahedron 2009, 65, 8191;
(c) Assadi, N.; Pogodin, S.; Agranat, I. Eur. J. Org. Chem. 2011, 6773;
(d) Nikolaev, V. A.; Ivanov, A. V.; Shakhmin, A. A.; Sieler, J.; Rodina, L. L. Tetrahedron Lett. 2012, 53, 3095;
(e) Nikolaev, V. A.; Ivanov, A. V.; Rodina, L. L.; Mlostoń, G. Beilstein J. Org. Chem. 2013, 9, 2751.
[38] Torres-Alacan, J.; Sander, W. J. Org. Chem. 2008, 73, 7118.
[39] Chen, J.-H.; Liu, S.-R.; Chen, K. Chem. Asian J. 2010, 5, 328.
[40] Chen, Z.; Fan, S.-Q.; Zheng, Y.; Ma, J.-A. Chem. Commun. 2015, 51, 16545.
[41] Wang, S.; Yang, L.-J.; Zeng, J.-L.; Zheng, Y.; Ma, J.-A. Org. Chem. Front. 2015, 2, 1468.
[42] (a) Saka?, M. N.; Gakovi?, A. R.; Csanádi, J. J.; Djurendi?, E. A.; Klisuri?, O.; Koji?, V.; Bogdanovi?, G.; Gaši, K. M. P. Tetrahedron Lett. 2009, 50, 4107.
(b) Mani, N. S.; Fitzgerald, A. E. J. Org. Chem. 2014, 79, 8889.
[43] (a) Supurgibekov, M. B.; Hennig, L.; Schulze, B.; Nikolaev, V. A. Russ. J. Org. Chem. 2008, 44, 1840;
(b) Supurgibekov, M. B.; Zakharova, V. M.; Sieler, J.; Nikolaev, V. A. Tetrahedron Lett. 2011, 52, 341;
(c) Muthusamy, S.; Srinivasan, P. Tetrahedron Lett. 2009, 50, 1331;
(d) Bel Abed, H.; Mammoliti, O.; Van Lommen, G.; Herdewijn, P. Tetrahedron Lett. 2012, 53, 6489;
(e) Bel Abed, H.; Mammoliti, O.; Bande, O.; Van Lommen, G.; Herdewijn, P. J. Org. Chem. 2013, 78, 7845;
(f) Bel Abed, H.; Mammoliti, O.; Bande, O.; Van Lommen, G.; Herdewijn, P. Org. Biomol. Chem. 2014, 12, 7159;
(g) Bel Abed, H.; Bande, O.; Mammoliti, O.; Van Lommen, G.; Herdewijn, P. Tetrahedron Lett. 2013, 54, 7056;
(h) Nikolaev, V. A.; Cantillo, D.; Kappe, C. O.; Medvedev, J. J.; Prakash, G. K. S.; Supurgibekov, M. B. Chem. Eur. J. 2016, 22, 174.
[44] Mao, H.; Lin, A.; Tang, Z.; Hu, H.; Zhu, C.; Cheng, Y. Chem. Eur. J. 2014, 20, 2454.
[45] (a) Yasui, E.; Wada, M.; Takamura, N. Tetrahedron Lett. 2009, 50, 4762;
(b) Yasui, E.; Wada, M.; Takamura, N. Tetrahedron 2009, 65, 461;
(c) Yasui, E.; Wada, M.; Nagumo, S.; Takamura, N. Tetrahedron 2013, 69, 4325.
[46] (a) van Berkel, S. S.; Brauch, S.; Gabriel, L.; Henze, M.; Stark, S.; Vasilev, D.; Wessjohann, L. A.; Abbas, M.; Westermann, B. Angew. Chem. Int. Ed. 2012, 51, 5343;
(b) Kuznetsov, A.; Gulevich, A. V.; Wink, D. J.; Gevorgyan, V. Angew. Chem. Int. Ed. 2014, 53, 9021.
[47] Li, L.; Chen, J.-J.; Li, Y.-J.; Bu, X.-B.; Liu, Q.; Zhao, Y.-L. Angew. Chem. Int. Ed. 2015, 54, 12107.
[48] Li, W.; Liu, X.; Hao, X.; Hu, X.; Chu, Y.; Cao, W.; Qin, S.; Hu, C.; Lin, L.; Feng, X. J. Am. Chem. Soc. 2011, 133, 15268.
[49] (a) Santos, F. M. F.; Rosa, J. N.; André, V.; Duarte, M. T.; Veiros, L. F.; Gois, P. M. P. Org. Lett. 2013, 15, 1760;
(b) António, J. P. M.; Frade, R. F. M.; Santos, F. M. F.; Coelho, J. A. S.; Afonso, C. A. M.; Gois, P. M. P.; Trindade, A. F. RSC Adv. 2014, 4, 29352.
[50] Mei, L.-Y.; Tang, X.-Y.; Shi, M. Chem. Eur. J. 2014, 20, 13136.
[51] Zheng, J.; Qi, J.; Cui, S. Org. Lett. 2016, 18, 128.
[52] Babinski, D. J.; Aguilar, H. R.; Still, R.; Frantz, D. E. J. Org. Chem. 2011, 76, 5915.
[53] Babinski, D. J.; Bao, X.; El Arba, M.; Chen, B.; Hrovat, D. A.; Borden, W. T.; Frantz, D. E. J. Am. Chem. Soc. 2012, 134, 16139.
[54] Guo, H.; Zhang, D.; Zhu, C.; Li, J.; Xu, G.; Sun, J. Org. Lett. 2014, 16, 3110.
[55] Xu, G.; Zhu, C.; Gu, W.; Li, J.; Sun, J. Angew. Chem. Int. Ed. 2015, 54, 883.
[56] Jordão, A. K.; Afonso, P. P.; Ferreira, V. F.; de Souza, M. C. B. V.; Almeida, M. C. B.; Beltrame, C. O.; Paiva, D. P.; Wardell, S. M. S. V.; Wardell, J. L.; Tiekink, E. R. T.; Damaso, C. R.; Cunha, A. C. Eur. J. Med. Chem. 2009, 44, 3777.
[57] Campos, V. R.; Abreu, P. A.; Castro, H. C.; Rodrigues, C. R.; Jordão, A. K.; Ferreira, V. F.; de Souza, M. C. B. V.; da C. Santos, F.; Moura, L. A.; Domingos, T. S.; Carvalho, C. Sanchez, E. F.; Fuly, A. L.; Cunha, A. C. Bioorg. Med. Chem. 2009, 17, 7429.
[58] Wang, Z.; Bi, X.; Liao, P.; Zhang, R.; Liang, Y.; Dong, D. Chem. Commun. 2012, 48, 7076.
[59] Deng, G.; Wang, F.; Lu, S.; Cheng, B. Org. Lett. 2015, 17, 4651.
[60] Zhou, L.; Liu, Z.; Liu, Y.; Zhang, Y.; Wang, J. Tetrahedron 2013, 69, 6083.
[61] González, A.; Pérez, D.; Puig, C.; Ryder, H.; Sanahuja, J.; Solé, L.; Bach, J. Tetrahedron Lett. 2009, 50, 2750.
[62] Hasegawa, K.; Kimura, N.; Arai, S.; Nishida, A. J. Org. Chem. 2008, 73, 6363.
[63] Barluenga, J.; Lonzi, G.; Riesgo, L.; Tomás, M.; López, L. A. J. Am. Chem. Soc. 2011, 133, 18138.
[64] Qiu, L.; Huang, D.; Xu, G.; Dai, Z.; Sun, J. Org. Lett. 2015, 17, 1810.
/
| 〈 |
|
〉 |