

Influence of Preparation Conditions of MoO3/C-N Hybrid Materials on Its Structure and Catalytic Performance
Received date: 2016-01-18
Online published: 2016-04-26
Supported by
Project supported by the Fundamental Research Funds for the Central Universities (Granted Number GK200902008).
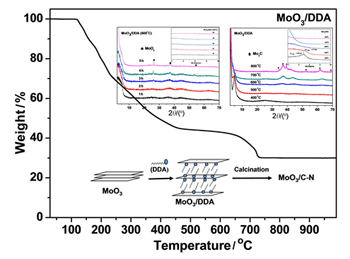
Molybdenum oxide (MoO3)/dodecylamine (DDA) intercalated materials were synthesized via direct thermal treatment followed by calcination to give MoO3/C-N hybrid materials. These prepared intercalated materials were characterized by powder X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy to investigate the influences of the calcination conditions, such as calcination temperature, calcination heating rate and calcination time, on the structure and composition of these materials. The results exhibited the order-disorder-order changes of the crystal structure during the calcination temperature from 400 ℃ to 800 ℃. Meanwhile, the valence of some Mo was reduced from +6 to +4 or +2. XRD patterns showed that calcination heating rate had almost no effect on the composite structure. Crystal MoO2 was produced with the increase of calcination time at 600 ℃ in N2 atmosphere. Crystal Mo2C was formed and the crystalline became regular with the increase of calcination temperature when the calcination temperature was higher than 600 ℃. SEM and TEM images clearly showed that molybdenum oxide layers were kept with the reducing of interlayer spacing as the calcination temperature below 600 ℃. With the calcination temperature rising up to 800 ℃, the carbonization effect of carbonaceous molecules and the enormous loss of gas molecules made the layer structure collapsed. In addition, the carbon and nitrogen elements were detected on the surface of molybdenum oxide. MoO3/C-N hybrid materials were used as catalyst for the oxidation of benzyl alcohol. The results showed that the structure and composition of the materials have a certain effect on the catalytic yield and the selectivity. The MoO3/C-N hybrid materials formed at calcination of 600 ℃ in 2 h was found to catalyze benzyl alcohol to benzaldehyde efficiently with high selectivity and relative stability. The yield of oxidation of benzyl alcohol to benzaldehyde in 3 h was up to 30% with a high selectivity retention, which was nearly 4 times compared with that of the pristine MoO3. The MoO3/C-N hybrid materials used as catalyst can be recycled several times with high selectivity.
Zhang Ting , Cai Xuediao , Liu Na , Xu Chunli . Influence of Preparation Conditions of MoO3/C-N Hybrid Materials on Its Structure and Catalytic Performance[J]. Acta Chimica Sinica, 2016 , 74(5) : 441 -449 . DOI: 10.6023/A16010036
[1] Faughnan, B. W.; Crandall, R. S. Appl. Phys. Lett. 1977, 31, 834.
[2] Yao, J. N.; Loo, B. H.; Hashimoto, K.; Fujishima, A. J. Electroanal. Chem. 1990, 290, 263.
[3] Yao, J. N.; Yang, Y. A.; Loo, B. H. J. Phys. Chem. B 1998, 102, 1856.
[4] Balendhran, S.; Deng, J.; Ou, J. Z.; Walia, S.; Scott, J.; Tang, J.; Wang, K. L.; Field, M. R.; Russo, S.; Zhuiykov, S.; Strano, M. S.; Medhekar, N.; Sriram, S.; Bhaskaran, M.; Kalantar-zadeh, K. Adv. Mater. 2013, 25, 109.
[5] Campanel, L.; Pistoia, G. J. Electrochem. Soc. 1971, 118, 1905.
[6] Zhang, Z.; Yang, R. Y.; Umar, A.; Gao, Y. S.; Wang, J. Y.; Lu, P.; Guo, Z. H.; Huang, L.; Zhou, T. T.; Wang, Q. Adv. Mater. Sci. 2014, 6(10), 2159.
[7] Brookes, C.; Wells, P. P.; Cibin, G.; Dimitratos, N.; Jones, W.; Morgan, D. J.; Bowker, M. ACS Catal. 2014, 4(1), 243.
[8] Shuwa, S. M.; Al-Hajri, R. S.; Jibril, B. Y.; Al-Waheibi, Y. M. Electron. Mater. Lett. 2015, 11(2), 252.
[9] Meyer, J.; Hamwi, S.; Kroger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Adv. Mater. 2012, 24, 5408.
[10] Alsaif, M. M. Y. A.; Balendhran, S.; Field, M. R.; Latham, K.; Wlodarski, W.; Ou, J. Z.; Kalantar-zadeh, K. Sens. Actuators, B: Chem. 2014, 192, 196.
[11] Suzuki, T.; Yamazaki, T.; Koukitu, A.; Maeda, M.; Seki, H.; Takahashi, K. J. Mater. Sci. Lett. 1988, 7, 926.
[12] Kamiya, S.; Tsuda, D.; Miura, K.; Sasaki, N. Wear 2004, 257, 1133.
[13] Sha, X. W.; Chen, L.; Cooper, A. C.; Pez, G. P.; Cheng, H. S. J. Phys. Chem. C 2009, 113, 11399.
[14] Dong, Y. F.; Xua, X. M.; Li, S.; Han, C. H.; Zhao, K. N.; Zhang, L.; Niu, C. J.; Huang, Z.; Mai, L. Q. Nano Energy 2015, 15, 145.
[15] Itoh, T.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M. Sens. Actuators, B 2008, 128, 512.
[16] Yao, D. D.; Ou, J. Z.; Latham, K.; Zhuiykov, S.; O’Mullane, A. P.; Kalantar-zadeh, K. Cryst. Growth Des. 2012, 12, 1865.
[17] Gesheva, K. A.; Ivanova, T. M.; Bodurov, G. Prog. Org. Coat. 2012, 74, 635.
[18] Mai, L. Q.; Hu, B.; Chen, W.; Qi, Y. Y.; Lao, C. S.; Yang, R. S.; Dai, Y.; Wang, Z. L. Adv. Mater. 2007, 19, 3712.
[19] Itoh, T.; Wang, J. Z.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M.; Murayama, N. Mater. Lett. 2008, 62, 3021.
[20] Itoh, T.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M. Mater. Chem. Phys. 2008, 110, 115.
[21] Murugana, A. V.; Viswanath, A. K. J. Appl. Phys. 2006, 100, 074319.
[22] Afsharpour, M.; Mahjoub, A. R.; Amini, M. M. J. Inorg. Organomet. Polym. 2009, 19, 298.
[23] Afsharpour, M.; Mahjoub, A. R.; Amini, M. M.; Khodadadi, A. A. Curr. Nanosci. 2010, 6, 82.
[24] Ji, H. M.; Liu, X. L.; Liu, Z. J.; Yan, B.; Chen, L.; Xie, Y. F.; Liu, C.; Hou, W. H.; Yang, G. Adv. Funct. Mater. 2015, 25, 1886.
[25] Qiu, J. Y. C.; Yang, Z. X.; Li, Y. J. Mater. Chem. A 2015, 3, 24245.
[26] Jing, Y.; Pan, Q. Y.; Cheng, Z. X.; Dong, X. W.; Xiang, Y. X. Mater. Sci. Eng. B 2007, 138, 55.
[27] Saghafi, M.; Ataie, A.; Heshmati-Manesh, S. Int. J. Refract. Met. Hard Mater. 2011, 29, 419.
[28] Ferrari, A. C.; Basko, D. M. Nature Nanotech. 2013, 46, 236.
[29] Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Nano Lett. 2007, 7, 238.
[30] Ferrari, A. C. Solid State Commun. 2007, 143, 47.
[31] Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K. S.; Basko, D. M.; Ferrari, A. C. Nano Lett. 2009, 9, 1433.
[32] Kudin, N. K.; Ozbas, B.; Schniepp, H. C.; Prud’homme, R. K.; Aksay, I. A.; Car, R. Nano Lett. 2008, 1, 36.
/
| 〈 |
|
〉 |