

Alloyed Pt–Ru Nanoparticles Immobilized on Mesostructured Nitrogen-Doped Carbon Nanocages for Efficient Methanol Electrooxidation
Received date: 2016-04-20
Online published: 2016-06-07
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2013CB932902), National Natural Science Foundation of China (Nos. 21473089, 51232003, 21373108, 51571110, 21573107), Suzhou Science and Technology Project (ZXG2013025) and Changzhou Technology Support Program (CE20130032). This work was also supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
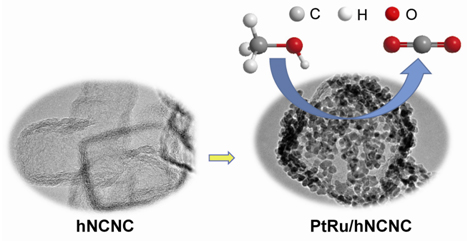
Direct methanol fuel cells (DMFC) have attracted extensive attention as ideal candidates for automotive and portable applications owing to the fascinating advantages such as high conversion efficiency, environmental friendliness, safety, wide sources of methanol, and simple cell structure. Electrocatalysts are one of crucial factors limiting the performance of DMFC. Nowadays, precious Pt-based catalyst, in spite of costliness and scarcity, is the most popular catalyst for methanol oxidation reaction (MOR) at anode due to the much better performances than those of the non-Pt catalysts. But there exists some shortcomings such as poor CO-tolerance and durability. Pt alloying with other metals, e.g. Ru, is an effective strategy to improve the catalytic performance. In addition, the support with a large specific surface area (SSA), high conductivity and suitable porous structure, such as sp2 carbon, could lead to high dispersion, high utilization and stability of Pt-based nanoparticles, also favorable for MOR. Recently, by in situ MgO template method, we reported the unique 3D hierarchical carbon-based nanocages featured with ultrahigh SSA, micro-meso-macro-pore coexistence, good conductivity and easy doping, which exhibited excellent electrochemical performances. Herein, taking the advantages of nitrogen-dopant anchoring function and unique mesostructures of hierarchical N-doped carbon nanocages (hNCNC), we report the Pt-Ru electrocatalysts immobilized on hNCNC (Pt-Ru/hNCNC) prepared via modified microwave-assisted ethylene glycol (EG) reduction method. The so-constructed Pt-Ru/hNCNC catalysts with ca. 30 wt% loading and tunable atomic ratio of Pt to Ru have a highly homogeneous dispersion of metal nanoparticles with the average size of ca. 3 nm. The alloying Pt-Ru/hNCNC catalysts demonstrate good CO-tolerance, high MOR activity and durability, superior to those of the counterparts of Pt/hNCNC and commercial PtRu/C. The good electrochemical performance can be ascribed to the synergistic effects of the bifunctional effect due to introduction of Ru, small size and high dispersion of metal nanoparticles induced by the large SSA and nitrogen participation of hNCNC, and multi-scaled hierarchical pore structures beneficial to the mass transportation. These results proposed a potential strategy to develop the high-performance Pt-based MOR catalysts based on the novel mesostructured hNCNC.
Li Danqin , Zhang Zhiqi , Zang Pengyuan , Ma Yanwen , Wu Qiang , Yang Lijun , Chen Qiang , Wang Xizhang , Hu Zheng . Alloyed Pt–Ru Nanoparticles Immobilized on Mesostructured Nitrogen-Doped Carbon Nanocages for Efficient Methanol Electrooxidation[J]. Acta Chimica Sinica, 2016 , 74(7) : 587 -592 . DOI: 10.6023/A16040196
[1] Chen, A.; Holt-Hindle, P. Chem. Rev. 2010, 110, 3767.
[2] Kakati, N.; Maiti, J.; Lee, S. H.; Jee, S. H.; Viswanathan, B.; Yoon, Y. S. Chem. Rev. 2014, 114, 12397.
[3] Gasteiger, H. A.; Markovic, N.; Ross, P. N.; Cairns, E. J. J. Phys. Chem. 1994, 98, 617.
[4] Liu, Z. L.; Guo, B.; Hong, L.; Lim, T. H. Electrochem. Commun. 2006, 8, 83.
[5] Pereira, L. G. S.; dos Santos, F. R.; Pereira, M. E.; Paganin, V. A.; Ticianelli, E. A. Electrochim. Acta 2006, 51, 4061.
[6] Sun, J.; Ma, H.; Jiang, H.; Dang, L.; Lu, Q.; Gao, F. J. Mater. Chem. 2015, 3, 15882.
[7] Mylswamy, S.; Wang, C. Y.; Liu, R. S.; Lee, J. F.; Tang, M. J.; Lee, J. J.; Weng, B. J. Chem. Phys. Lett. 2005, 412, 444.
[8] Watanabe, M.; Motoo, S. J. Electroanal. Chem. Interfacial Electrochem. 1975, 60, 267.
[9] Yue, B.; Ma, Y. W.; Tao, H. S.; Yu, L. S.; Jian, G. Q.; Wang, X. Z.; Wang, X. S.; Lu, Y. N.; Hu, Z. J. Mater. Chem. 2008, 18, 1747.
[10] Jiang, S.; Zhu, L.; Ma, Y.; Wang, X.; Liu, J.; Zhu, J.; Fan, Y.; Zou, Z.; Hu, Z. J. Power Sources 2010, 195, 7578.
[11] Feng, H.; Ma, J.; Hu, Z. J. Mater. Chem. 2010, 20, 1702.
[12] Joo, S. H.; Kwon, K.; You, D. J.; Pak, C.; Chang, H.; Kim, J. M. Electrochim. Acta 2009, 54, 5746.
[13] Guerrero-Ruiz, A.; Badenes, P.; Rodriguez-Ramos, I. Appl. Catal. A: Gen. 1998, 173, 313.
[14] Kuang, Y.; Cui, Y.; Zhang, Y.; Yu, Y.; Zhang, X.; Chen, J. Chem. Eur. J. 2012, 18, 1522.
[15] Che, G.; Lakshmi, B. B.; Fisher, E. R.; Martin, C. R. Nature 1998, 393, 346.
[16] Lin, M. L.; Huang, C. C.; Lo, M. Y.; Mou, C. Y. J. Phys. Chem. C 2008, 112, 867.
[17] Liu, Z.; Su, F.; Zhang, X.; Tay, S. W. ACS Appl. Mater. Interfaces 2011, 3, 3824.
[18] Yu, J. S.; Kang, S.; Yoon, S. B.; Chai, G. J. Am. Chem. Soc. 2002, 124, 9382.
[19] Li, F.; Chan, K.-Y.; Yung, H.; Yang, C.; Ting, S. W. Phys. Chem. Chem. Phys. 2013, 15, 13570.
[20] Cong, H.-P.; Ren, X.-C.; Yu, S.-H. ChemCatChem 2012, 4, 1555.
[21] Bin, D.; Ren, F.; Wang, H.; Zhang, K.; Yang, B.; Zhai, C.; Zhu, M.; Yang, P.; Du, Y. RSC Adv. 2014, 4, 39612.
[22] La-Torre-Riveros, L.; Guzman-Blas, R.; Méndez-Torres, A. E.; Prelas, M.; Tryk, D. A.; Cabrera, C. R. ACS Appl. Mater. Interfaces 2012, 4, 1134.
[23] Jiang, S.; Ma, Y.; Jian, G.; Tao, H.; Wang, X.; Fan, Y.; Lu, Y.; Hu, Z.; Chen, Y. Adv. Mater. 2009, 21, 4953.
[24] Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Adv. Mater. 2012, 24, 5593.
[25] Xie, K.; Qin, X.; Wang, X.; Wang, Y.; Tao, H.; Wu, Q.; Yang, L.; Hu, Z. Adv. Mater. 2012, 24, 347.
[26] Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. ACS Catal. 2015, 5, 6707.
[27] Lyu, Z.; Xu, D.; Yang, L.; Che, R.; Feng, R.; Zhao, J.; Li, Y.; Wu, Q.; Wang, X.; Hu, Z. Nano Energy 2015, 12, 657.
[28] Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; Liu, J.; Hu, Z. Adv. Mater. 2015, 27, 3541.
[29] Lyu, Z.; Feng, R.; Zhao, J.; Fan, H.; Xu, D.; Wu, Q.; Yang, L.; Chen, Q.; Wang, X.; Hu, Z. Acta Chim. Sinica 2015, 73, 1013. (吕之阳, 冯瑞, 赵进, 范豪, 徐丹, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2015, 73, 1013.)
[30] Feng, R.; Wang, L.; Lyu, Z.; Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Acta Chim. Sinica 2014, 72, 653. (冯瑞, 王立伟, 吕之阳, 吴强, 杨立军, 王喜章, 胡征, 化学学报, 2014, 72, 653.)
[31] Prabhuram, J.; Zhao, T. S.; Liang, Z. X.; Chen, R. Electrochim. Acta 2007, 52, 2649.
[32] Roth, C.; Benker, N.; Theissmann, R.; Nichols, R. J.; Schiffrin, D. J. Langmuir 2008, 24, 2191.
[33] Bock, C.; Paquet, C.; Couillard, M.; Botton, G. A.; MacDougall, B. R. J. Am. Chem. Soc. 2004, 126, 8028.
[34] Giorgi, L.; Pozio, A.; Bracchini, C.; Giorgi, R.; Turtù, S. J. Appl. Electrochem. 2001, 31, 325.
[35] Wang, Z.-C.; Ma, Z.-M.; Li, H.-L. Appl. Surf. Sci. 2008, 254, 6521.
[36] Liu, S.-H.; Yu, W.-Y.; Chen, C.-H.; Lo, A.-Y.; Hwang, B.-J.; Chien, S.-H.; Liu, S.-B. Chem. Mater. 2008, 20, 1622.
/
| 〈 |
|
〉 |