

Reaction-Based Ratiometric Fluorescence Probes For Dopamine Detection
Received date: 2016-04-06
Online published: 2016-07-19
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21206122, 21233011).
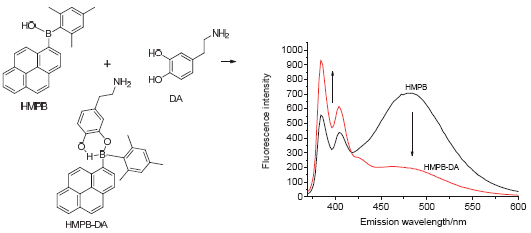
Dopamine (DA) is one of neurotransmitters in the human central nervous system and plays a very important role on human behavior and brain function. It is necessary to detect dopamine and determine its concentration in organism. Of all the dopamine detection methods reported, fluorescence probes exhibit high efficiency, considerate specifity and potential for real-time detection. Considering the high quantum field and strong trend to form an excimer of pyrene fluorophore, a fluorescent probe hydroxy(mesityl)(pyren-1-yl)borane (HMPB) based on pyrenyl boron compound was designed, synthesized and utilized in dopamine detection. In the phosphate buffer solution (PBS) with the aid of surfactant decyltrimethylammonium bromide, the water-insoluble HMPB could show two fluorescent bands: a monomer band of peaked at 388 nm and an excimer band peaked at 484 nm. The solubility of HMPB improved after addition of DA because it could react with HMPB to form a new compound HMPB-DA and it is better to dissolve in water. The excimer pyrenyl, therefore, gradually dissociated to generate monomer pyrenyl. Accordingly, as the addition of DA, fluorescent intensity at 484 nm (I484 nm) decreased, while intensity at 388 nm (I388 nm) increased. Within 10 min, the fluorescence intensity reached saturation and the ratio of I388 nm to I484 nm have great changed after adding dopamine. HMPB exhibited significant fluorescent response when the concentration range of DA is 10 nmol/L to 600 nmol/L in 10 min, which matched the physiologic concentration of DA. The ratio of I388 nm to I484 nm could be utilized to determine the concentration of dopamine. The detection limit of HMPB was as low as 14.6 nmol/L. HMPB showed no response to common amino acids, saccharides, proteins, ions, catecholamin and other bioactive molecules, even if the interference molecules were at high concentrations. Meanwhile, HMPB can be used to detect dopamine in urine of organisms. With fast response, high sensitivity and accuracy, HMPB represented considerable potential to act as a DA detector in physiological environment, which could become a promising tool in biochemistry, molecular biology and diagnostics investigation.
Key words: dopamine; fluorescent probe; boron compound; spectrofluorimetry; high selectivity
Zhao Zhensheng , Guo Xudong , Li Shayu , Yang Guoqiang . Reaction-Based Ratiometric Fluorescence Probes For Dopamine Detection[J]. Acta Chimica Sinica, 2016 , 74(7) : 593 -596 . DOI: 10.6023/A16030160
[1] (a) Robinson, D. L.; Hermans, A.; Seipel, A. T.; Wightman, R. M. Chem. Rev. 2008, 108, 2554;
(b) Hyman, S. E.; Malenka, R. C. Nat. Rev. Neurosci. 2001, 2, 695.
[2] Li, B.-R.; Hsieh, Y.-J.; Chen, Y.-X.; Chung, Y.-T.; Pan, C.-Y.; Chen, Y.-T. J. Am. Chem. Soc. 2013, 135, 16034.
[3] Li, L.; Liu, H.; Shen, Y.; Zhang, J.; Zhu, J. J. Anal. Chem. 2011, 83, 661.
[4] (a) Tyagi, P.; Postetter, D.; Saragnese, D.; Randall, C.; Mirski, M.; Gracias, D. Anal. Chem. 2009, 81, 9979;
(b) Koehne, J. E.; Marsh, M.; Boakye, A.; Douglas, B.; Kim, I. Y.; Chang, S.-Y.; Jang, D.-P.; Bennet, K. E.; Kimble, C.; Andrews, R. Analyst 2011, 136, 1802;
(c) Zhou, J.; Wang, W.; Yu, P.; Xiong, E.; Zhang, X.; Chen, J. RSC. Adv 2014, 4, 52250;
(d) Liu, Q.; Zhu, X.; Huo, Z.; He, X.; Liang, Y.; Xu, M. Talanta 2012, 97, 557;
(e) Wei, X.; Chang, C.; Li, J. Acta. Chim. Sinica 2013, 71, 951 (in Chinese). (魏小平, 常川, 李建平, 化学学报, 2013, 71, 951.)
[5] Zhao, Y.; Zhao, S.; Huang, J.; Ye, F. Talanta 2011, 85, 2650.
[6] Liu, L.; Li, S.; Liu, L.; Deng, D.; Xia, N. Analyst 2012, 137, 3794.
[7] (a) Lee, H.-C.; Chen, T.-H.; Tseng, W.-L.; Lin, C.-H. Analyst 2012, 137, 5352;
(b) Su, H.; Sun, B.; Chen, L.; Xu, Z.; Ai, S. Anal. Methods 2012, 4, 3981.
[8] Nikolajsen, R. P.; Hansen, Å. M. Anal. Chim. Acta 2001, 449, 1.
[9] Zhang, L.; Teshima, N.; Hasebe, T.; Kurihara, M.; Kawashima, T. Talanta 1999, 50, 677.
[10] (a) Ma, Y.; Yang, C.; Li, N.; Yang, X. Talanta 2005, 67, 979;
(b) Mu, Q.; Xu, H.; Li, Y.; Ma, S.; Zhong, X. Analyst 2014, 139, 93;
(c) Zhao, D.; Song, H.; Hao, L.; Liu, X.; Zhang, L.; Lv, Y. Talanta 2013, 107, 133;
(d) Yu, C.; Luo, M.; Zeng, F.; Zheng, F.; Wu, S. Chem. Commun. 2011, 47, 9086.
[11] (a) Jackowska, K.; Krysinski, P. Anal. Bioanal. Chem. 2013, 405, 3753;
(b) She, G.; Huang, X.; Jin, L.; Qi, X.; Mu, L.; Shi, W. Small 2014, 10, 4685.
[12] Feng, J.; Tian, K.; Hu, D.; Wang, S.; Li, S.; Zeng, Y.; Li, Y.; Yang, G. Angew. Chem., Int. Ed. 2011, 50, 8072.
/
| 〈 |
|
〉 |