

First-Principles Theory Investigation on Structural and Photoelectronic Properties of Formamidinium Lead Halide Perovskites
Received date: 2016-05-17
Online published: 2016-08-10
Supported by
Project supported by the National Natural Science Foundation of China (21303266), Postgraduate's Innovation Project (YCXJ2016084) and Student's Platform for Innovation and Entrepreneurship Training Program (No. 20151342)
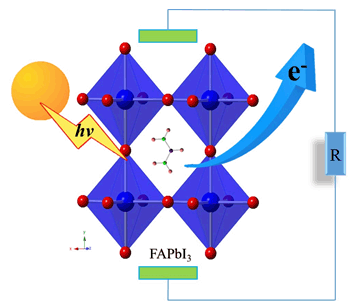
Formamidinium lead halide perovskites have attracted wide attention as photoelectronic conversion materials due to the high photoelectronic conversion efficiency (PCE), low cost and simple synthetic process. The structural, electronic and optical properties of mixed formamidinium lead halide perovskites FAPbIxCl3-x (FA=NH2CH=NH2+, x=0~3) have been investigated by the first-principles theory. Our results show that FA cations lie along [001] direction in the trigonal FAPbX3 (X=Cl, Br, I). However, the direction is slightly shifted owing to the distortion of PbX6 (X=Cl, I) octahedrons in the mixed FAPbIxCl3-x. The Pb-I bond distances (0.315~0.334 nm) are larger than Pb-Cl bond distances (0.282~0.302 nm). With the increase of I/Cl ratio, the lattice parameters and volumes of FAPbIxCl3-x increase. The FA cations play a crucial role in balancing the crystal structure, but they do not participate into the process of frontier orbital transition directly. They just play the role of charge donors to contribute ca. 0.76 e to PbI3 framework. FAPbIxCl3-x are direct band-gap semiconductors, with the direct bandgap nature at Z (0, 0, 0.5) symmetry point. The valence band maximum (VBM) is composed of antibonding orbitals of I 5p (Cl 3p) and a few Pb 6s orbitals, and the conduction band minimum (CBM) is composed of Pb 6p orbital. There exists a combined covalent and ionic bonding mechanism between Pb and I (Cl) ions. As the I/Cl ratio increases, the band gaps decrease and the absorption spectra are red shifted. FAPbI3 has an ideal band gap of 1.53 eV. It exhibits the superior absorption spectrum especially in the range of 300 nm to 500 nm, which elucidates that FAPbI3 has great potential as the photoelectronic conversion material. Our results could provide theoretical guidance for the experimental design and synthesis of perovskite solar cells.
Zhao Zigang , Niu Yongqiang , Zhao Yang , Song Qinghua , Xin Ling , Lu Xiaoqing . First-Principles Theory Investigation on Structural and Photoelectronic Properties of Formamidinium Lead Halide Perovskites[J]. Acta Chimica Sinica, 2016 , 74(8) : 689 -693 . DOI: 10.6023/A16050245
[1] Snaith, H. J. J. Phys. Chem. Lett. 2013, 4, 3623.
[2] Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. J. Am. Chem. Soc. 2009, 131, 6050.
[3] Kim, H. S.; Lee, C. R.; Im, J. H.; Lee, K. B.; Moehl, T.; Marchioro, A.; Moon, S. J.; Humphry-Baker, R.; Yum, J. H.; Moser, J. E.; Gratzel, M.; Park, N. G. Sci. Rep. 2012, 2, 591.
[4] Burschka, J.; Pellet, N.; Moon, S. J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M. K.; Gratzel, M. Nature 2013, 499, 316.
[5] Lee, M. M.; Teuscher, J.; Miyasaka, T.; Murakami, T. N.; Snaith, H. J. Science 2012, 338, 643.
[6] You, J.; Hong, Z.; Yang, Y.; Chen, Q.; Cai, M.; Song, T. B.; Chen, C. C.; Lu, S.; Liu, Y.; Zhou, H.; Yang, Y. ACS Nano 2014, 8, 1674.
[7] Wehrenfennig, C.; Eperon, G. E.; Johnston, M. B.; Snaith, H. J.; Herz, L. M. Adv. Mater. 2014, 26, 1584.
[8] Gao, P.; Gratzel, M.; Nazeeruddin, M. K. Energy Environ. Sci. 2014, 7, 2448.
[9] Boix, P. P.; Nonomura, K.; Mathews, N.; Mhaisalkar, S. G. Mater. Today 2014, 17, 16.
[10] Noh, J. H.; Im, S. H.; Heo, J. H.; Mandal, T. N.; Seok, S. I. Nano Lett. 2013, 13, 1764.
[11] Im, J. H.; Chung, J.; Kim, S. J.; Park, N. G. Nanoscale Res. Lett. 2012, 7, 1.
[12] Koh, T. M.; Fu, K.; Fang, Y.; Chen, S.; Sum, T. C.; Mathews, N.; Mhaisalkar, S. G.; Boix, P. P.; Baikie, T. J. Phys. Chem. C 2014, 118, 16458.
[13] Lv, S.; Pang, S.; Zhou, Y.; Padture, N. P.; Hu, H.; Wang, L.; Zhou, X.; Zhu, H.; Zhang, L.; Huang, C.; Cui, G. Phys. Chem. Chem. Phys. 2014, 16, 19206.
[14] Yang, W. S.; Noh, J. H.; Jeon, N. J.; Kim, Y. C.; Ryu, S.; Seo, J.; Seok, S. I. Science 2015, 348, 1234.
[15] Kresse, G.; Furthmüller, J. Comp. Mater. Sci. 1996, 6, 15.
[16] Monkhorst, H. J.; Pack, J. D. Phys. Rev. B 1976, 13, 5188.
[17] Even, J.; Pedesseau, L.; Jancu, J. M.; Katan, C. J. Phys. Chem. Lett. 2013, 4, 2999.
[18] Mosconi, E.; Amat, A.; Nazeeruddin, M. K.; Grätzel, M.; De Angelis, F. J. Phys. Chem. C 2013, 117, 13902.
[19] Goldschmidt, V. M. Naturwissenschaften 1926, 14, 477.
[20] Guo, X.; Niu, G.; Wang, L. Acta Chim. Sinica 2014, 73, 211. (郭旭东, 牛广达, 王立铎, 化学学报, 2014, 73, 211.)
[21] Yao, X.; Ding, Y.; Zhang, X.; Zhao, Y. Acta Phys. Sin. 2015, 64, 38805. (姚鑫, 丁艳丽, 张晓丹, 赵颖, 物理学报, 2015, 64, 38805.)
[22] McKinnon, N. K.; Reeves, D. C.; Akabas, M. H. J. Gen. Physiol. 2011, 138, 453.
[23] Green, M. A.; Ho-Baillie, A.; Snaith, H. J. Nat. Photon. 2014, 8, 506.
[24] Eperon, G. E.; Stranks, S. D.; Menelaou, C.; Johnston, M. B.; Herz, L.; Snaith, H. Energy Environ. Sci. 2014, 7, 982.
[25] Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. Chem. Mater. 2014, 26, 1485.
[26] Lee, J. W.; Seol, D. J.; Cho, A. N.; Park, N. G. Adv. Mater. 2014, 26, 4991.
[27] Yuan, J.; Gao, B.; Wang, W.; Wang, J. Acta Phys.-Chim. Sin. 2015, 31, 1302. (袁俊辉, 高博, 汪文, 王嘉赋, 物理化学学报, 2015, 31, 1302.)
/
| 〈 |
|
〉 |