

Molecular Design of Benzothiadiazole Derivatives Electron Acceptors and Matching of Donor-Acceptor Materials
Received date: 2016-05-30
Online published: 2016-08-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21173139, 21473108) and Shaanxi Innovative Team of Key Science and Technology (No. 2013KCT-17).
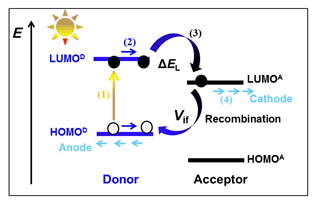
To better understand the relationships between the microstructure and the optoelectronic characteristics of the electron acceptor and to meet the needs of donor-acceptor materials with excellent optical properties for solar cell, a series of acceptor molecules with A'-π-A-π-A' type are designed. In these molecules, the core framework of benzothiadiazole is used as an acceptor (A), three kinds of conjugated heterocyclics (A') with different abilities of electron-withdrawing and steric effects are applied as the terminals, and various conjugated structures, such as the double bond, thiophene, benzothiophene and vinyl thiophene, are utilized as π-bridge, respectively. Their geometric configurations, the characteristics of frontier molecular orbital, optical properties, as well as the electronic reorganization energy are predicted by DFT-B3LYP and TD-DFT-CAM-B3LYP. Solvent effects from acetone and chlorobenzene on molecular properties are studied. Furthermore, the Donor-Acceptor (D-A) interfaces are respectively constructed by combining the excellent acceptors with the selected two donors. The DFT-D3 method is used to scan the binding energy of D-A complex, in order to determine the stacked displacement of the interface. The degree of interface recombination is evaluated by calculating electronic coupling (Vif) between HOMO of donors and LUMO of acceptors. The results show that modifying benzothiadiazole with a reasonable substituent is an effective way to adjust LUMO energy levels and lead to the noticeable variation of the energy gap. Combining planar electron acceptor materials (A'-π-A-π-A' type) with non-planar electron donor materials (D), to form the optical active layer is a practical approach for preventing interface recombination and achieving high open-circuit voltage (Voc). Considering ΔEL, Vif, light absorption efficiency, and solvation effect, D1-1aγ and D1-2aγ combinations are the most promising candidates of optical active layer materials in organic solar cell.
Shao Rong , Yang Xinbo , Yin Shiwei , Wang Wenliang . Molecular Design of Benzothiadiazole Derivatives Electron Acceptors and Matching of Donor-Acceptor Materials[J]. Acta Chimica Sinica, 2016 , 74(8) : 676 -682 . DOI: 10.6023/A16050268
[1] Sariciftci, N. S.; Smilowitz, L.; Heeger, A. J.; Wudl, F. Science 1992, 258, 1474.
[2] Anthony, J. E.; Facchetti, A.; Heeney, M.; Marder, S. R.; Zhan, X. Adv. Mater. 2010, 22, 3876.
[3] Meng, Q. B. Acta Chim. Sinica 2015, 73, 161. (孟庆波, 化学学报, 2015, 73, 161.)
[4] Carlotto, S. J. Phys. Chem. A 2014, 118, 4808.
[5] Armin, A.; Kassal, I.; Shaw, P. E.; Hambsch, M.; Stolterfoht, M.; Lyons, D. M.; Li, J.; Shi, Z.; Burn, P. L.; Meredith, P. J. Am. Chem. Soc. 2014, 136, 11465.
[6] Mishra, A.; Bäuerle, P. Angew. Chem. Int. Ed. 2012, 51, 2020.
[7] Walker, B.; Liu, J.; Kim, C.; Welch, G. C.; Park, J. K.; Lin, J.; Zalar, P.; Proctor, C. M.; Seo, J. H.; Bazan, G. C.; Nguyen, T.-Q. Energy Environ. Sci. 2013, 6, 952.
[8] Wang, Z.; Uemura, Y.; Zhou, Y.; Miyadera, T.; Azumi, R.; Yoshida, Y.; Chikamatsu, M. ACS Appl. Mater. Interfaces 2015, 7, 10814.
[9] Heremans, P.; Cheyns, D.; Rand, B. P. Acc. Chem. Res. 2009, 42, 1740.
[10] Reese, M. O.; Nardes, A. M.; Rupert, B. L.; Larsen, R. E.; Olson, D. C.; Lloyd, M. T.; Shaheen, S. E.; Ginley, D. S.; Rumbles, G.; Kopidakis, N. Adv. Funct. Mater. 2010, 20, 3476.
[11] Fu, Y.; Wang, F.; Zhang, Y.; Fang, X.; Lai, W.; Huang, W. Acta Chim. Sinica 2014, 72, 158. (付钰, 王芳, 张燕, 方旭, 赖文勇, 黄维, 化学学报, 2014, 72, 158.)
[12] Dou, C.; Chen, D.; Iqbal, J.; Yuan, Y.; Zhang, H.; Wang, Y. Langmuir 2011, 27, 6323.
[13] Woo, C. H.; Holcombe, T. W.; Unruh, D. A.; Sellinger, A. Chem. Mater. 2010, 22, 1673.
[14] Wolfer, P.; Schwenn, P. E.; Pandey, A. K.; Fang, Y.; Stingelin, N.; Burn, P. L.; Meredith, P. J. Mater. Chem. A. 2013, 1, 5989.
[15] Bloking, J. T.; Han, X.; Higgs, A. T.; Kastrop, J. P.; Pandey, L.; Norton, J. E.; Risko, C.; Chen, C. E.; Bredas, J.-E.; McGehee, M. D.; Sellinger, A. Chem. Mater. 2011, 23, 5484.
[16] Barone, V.; Cossi, M. J. Phys. Chem. A 1998, 102, 1995.
[17] Schlenker, C. W.; Thompson, M. E. Chem. Commun. 2011, 47, 3702.
[18] Newton, M. D.; Sutin, N. Ann. Rev. Phys. Chem. 1984, 35, 437.
[19] Barbara, P. F.; Meyer, T. J.; Ratner, M. A. J. Phys. Chem. 1996, 100, 13148.
[20] Lemaur, V.; Steel, M.; Beljonne, D.; Brédas, J.-L.; Cornil, J. J. Am. Chem. Soc. 2005, 127, 6077.
[21] Hsu, C. P. Acc. Chem. Res. 2009, 42, 509.
[22] Yang, Y. M.; Yin, S. W.; Li, L. L.; Yang, J. Y. Acta Chim. Sinica 2011, 69, 1991. (杨永梅, 尹世伟, 李兰兰, 杨家瑜, 化学学报, 2011, 69, 1991.)
[23] Grimme, S. WIREs Comput. Mol. Sci. 2011, 1, 211.
[24] Liu, H.; Brémond, É.; Prlj, A.; Gonthier, J. F.; Corminboeuf, C. J. Phys. Chem. Lett. 2014, 5, 2320.
[25] Guan, L.; Wang, W. L.; Shao, R.; Liu, F. Y.; Yin, S. W. J. Mol. Model. 2015, 21, 126-1.
[26] Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T. J.; Ratner, M. A.; Wasielewski, M. R.; Marder, S. R. Adv. Mater. 2011, 23, 268.
[27] Shao, R.; Wang, W. L.; Yang, X. B.; Yin, X. W. Sci. China-Chem. 2016, 46, 699. (邵绒,王文亮, 杨鑫博, 尹世伟, 中国科学: 化学, 2016, 46, 699.)
[28] Liu, X.; Su, S. J.; Cao, Y. Polymer Bulletin 2014, 12, 68. (刘欣, 苏仕健, 曹镛, 高分子通报, 2014, 12, 68.)
[29] Nalwa, H. S. Handbook of Advanced Electronic and Photonic Materials and Device, Academic San Diego, CA, 2001.
[30] Fitzner, R.; Reinold, E.; Mishra, A. Adv. Funct. Mater. 2011, 21, 897.
[31] Lin, L. Y.; Chen, Y. H.; Huang, Z. Y.; Lin, H. W.; Chou, S. H.; Lin, F.; Chen, C. W.; Liu, Y. H.; Wong, K. T. J. Am. Chem. Soc. 2011, 133, 15822.
/
| 〈 |
|
〉 |