

Palladium Catalyzed Arylation of C(sp3)-H Bonds of Carbonyl β-position in Water
Received date: 2016-07-11
Online published: 2016-09-06
Supported by
Project supported by the National Natural Science Foundation of China (grant Nos. 21272161, 21472128, J1310008).
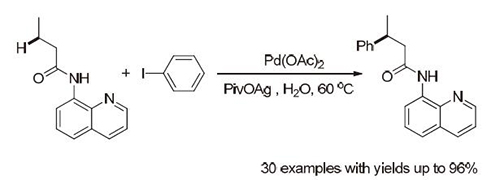
The direct activation and functionalization of C-H bonds is fundamentally important in organic synthesis. Among different methods developed, transition metal-catalyzed intermolecular arylation of alkanes, which couples unactivated C(sp3)-H bonds with aryl moieties, is recognized as one of the most powerful strategies to construct valuable arylated alkyl scaffolds. Tremendous progress has thus been made in this field, which usually require harsh reaction conditions such as high temperature and inert atmosphere as well as additives. Furthermore, the reaction media were usually organic solvents with undesirable toxicity and volatility, such as toluene, xylene, tert-amyl alcohol, dichloroethane, etc. Therefore, the development of efficient catalytic unactivated C(sp3)-H arylation under mild reaction conditions is still highly demanded. Herein, we reported a general and practical palladium-catalyzed arylation of β-methylene C(sp3)-H under aqueous conditions by the use of 8-aminoquinoline as directing groups. This method exhibited good to excellent yields up to 96% and good functional group tolerance without other additives and inert gas atmosphere. Meanwhile, the reaction showed good regioselectivity to the β-position of carbonyl group. Mechanism studies showed that the aliphatic Ag-carboxylate salt was critical for this reaction. The silver ion might weaken the C-I bond and function as halogen scavenger for the transformation, while pivalic acid ion might act as base during reaction. A representative procedure for this reaction is as following: To a 10 mL glass tube, N-(quinolin-8-yl) butyramide (42.8 mg, 0.2 mmol), iodobenzene (67 μL, 0.4 mmol), Pd(OAc)2 (4.5 mg), AgPiv (83.6 mg, 0.4 mmol) in 0.4 mL H2O were stirred at 60℃ for 24 h, and then cooled to room temperature. The reaction mixture was extracted with EtOAc. The organic phase was washed with water, dried over magnesium sulfate, and concentrated. The crude product was purified with column chromatography (petroleum/EtOAc) to provide the products in 61%~96% yields.
Key words: aqueous; palladium catalysis; 8-aminoquinoline; C-H activation; arylation
Luo Feihua , Long Yang , Li Zhengkai , Zhou Xiangge . Palladium Catalyzed Arylation of C(sp3)-H Bonds of Carbonyl β-position in Water[J]. Acta Chimica Sinica, 2016 , 74(10) : 805 -810 . DOI: 10.6023/A16060316
[1] (a) Yu, J.; Ding, K. Acta Chim. Sinica 2015, 73, 1223. (余金权, 丁奎岭, 化学学报, 2015, 73, 1223.); (b) Yuan, Y.; Song, S.; Jiao, N. Acta Chim. Sinica 2015, 73, 1231. (袁逸之, 宋颂, 焦宁, 化学学报, 2015, 73, 1231.); (c) Zhao, J.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235. (赵金钵, 张前, 化学学报, 2015, 73, 1235.)
[2] (a) Daugulis, O.; Do, H. Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074; (b) Jazzar, R.; Hitce, J.; Renaudat, A.; Sofack-Kreutzer, J.; Baudoin, O. Chem.-Eur. J. 2010, 16, 2654; (c) Wasa, M.; Engle, K. M.; Yu, J.-Q. Isr. J. Chem. 2010, 50, 605; (d) Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1, 191; (e) Baudoin, O. Chem. Soc. Rev. 2011, 40, 4902; (f) Gutekunst, W. R.; Baran, P. S. Chem. Soc. Rev. 2011, 40, 1976; (g) Rouquet, G.; Chatani, N. Angew. Chem. Int. Ed. 2013, 52, 11726; (h) Zhang, B.; Guan, H.; Liu, B.; Shi, B. Chin. J. Org. Chem. 2014, 34, 1487. (张博, 管晗曦, 刘斌, 史炳锋, 有机化学, 2014, 34, 1487.); (i) Zhou, L.; Lu, W. Acta Chim. Sinica 2015, 73, 1250. (周励宏, 陆文军, 化学学报, 2015, 73, 1250.); (j) Liao, G.; Shi, B. Acta Chim. Sinica 2015, 73, 1283. (廖港, 史炳锋, 化学学报, 2015, 73, 1283.)
[3] (a) Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154; (b) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132, 3965; (c) Nadres, E. T.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 7.
[4] Tran, L. D.; Daugulis, O. Angew. Chem. Int. Ed. 2012, 51, 5188.
[5] (a) Xie, Y. J.; Yang, Y. Z.; Huang, L. H.; Zhang, X. B.; Zhang, Y. H. Org. Lett. 2012, 1238; (b) He, G.; Chen, G. Angew. Chem. Int. Ed. 2011, 50, 5192.
[6] Rodriguez, N.; Revilla, R. J. A.; FernandezIbanez, M. A.; Carretero, J. C. Chem. Sci. 2013, 4, 175.
[7] (a) Zhang, Q.; Chen, K.; Rao, W.-H.; Zhang, Y.-J.; Chen, F.-J.; Shi, B. F. Angew. Chem. Int. Ed. 2013, 52, 13588; (b) Chen, F. J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S. Q.; Shi, B. F. Chem. Sci. 2013, 4, 4187; (c) Zhang, Q.; Yin, X. S.; Zhao, S.; Fang, S. L.; Shi, B. F. Chem. Commun. 2014, 50, 8353; (d) Chen, K.; Zhang, S. Q.; Jiang, H. Z.; Xu, J. W.; Shi, B. F. Chem. Eur. J. 2015, 21, 3264; (e) Yan, S. Y.; Liu, Y. J.; Liu, B.; Liu, Y. H.; Shi, B. F. Chem. Commun. 2015, 51, 4069; (f) Rao, W. H.; Shi, B. F. Org. Lett. 2015, 17, 2784. (g) Zhang, Q.; Yin, X. S.; Chen, K.; Zhang, S. Q.; Shi, B. F. J. Am. Chem. Soc. 2015, 137, 8219.
[8] Fan, M. Y.; Ma, D. W. Angew. Chem. Int. Ed. 2013, 52, 12152.
[9] (a) Wasa, M.; Chan, K. S. L.; Zhang, X. G.; He, J.; Miura, M.; Yu, J. Q. J. Am. Chem. Soc. 2012, 134, 18570; (b) He, J.; Wasa, M.; Chan, K. S. L.; Yu, J. Q. J. Am. Chem. Soc. 2013, 135, 3387; (c) He, J.; Li, S. H.; Deng, Y. Q.; Fu, H. Y.; Laforteza, B. N.; Spangler, J. E.; Homs, A.; Yu, J. Q. Science 2014, 343, 1216; (d) Li, G.; Wan, L.; Zhang, G. F.; Leow, D. S.; Spangler, J.; Yu, J. Q. J. Am. Chem. Soc. 2015, 137, 4391; (e) He, J.; Shigenari, T.; Yu, J. Q. Angew. Chem. Int. Ed. 2015, 54, 6545; (f) Zhu, R. Y.; Tanaka, K.; Li, G. C.; He, J.; Fu, H. Y.; Li, S. H.; Yu, J. Q. J. Am. Chem. Soc. 2015, 137, 7067.
[10] (a) Ye, X. H.; He, Z. R.; Ahmed, T.; Weise, K.; Akhmedov, N. G.; Petersena, J. L.; Shi, X. D. Chem. Sci. 2013, 4, 3712; (b) Song, W.; Lackner, S.; Ackermann, L. Angew. Chem. Int. Ed. 2014, 53, 2477.
[11] (a) Fischmeister, C.; Doucet, H. Green Chem. 2011, 13, 741; (b) Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437; (c) Simon, M. O.; Li, C. J. Chem. Soc. Rev. 2012, 41, 1415; (d) Li, B.; Dixneuf, P. H. Chem. Soc. Rev. 2013, 42, 5744.
[12] Selected examples: (a) Turner, G. L.; Morris, J. A.; Greaney, M. F. Angew. Chem. Int. Ed. 2007, 46, 7996; (b) Flegeau, E. F.; Popkin, M. E.; Greaney, M. F. Org. Lett. 2008, 10, 2717; (c) Ohnmacht, S. A.; Mamone, P.; Culshaw, A. J.; Greaney, M. F. Chem. Commun. 2008, 1241; (d) Ohnmacht, S. A.; Culshaw, A. J.; Greaney, M. F. Org. Lett. 2010, 12, 224; (e) Ruiz-Rodrguez, J.; Albericio, F.; Lavilla, R. Chem. Eur. J. 2010, 16, 1124; (f) Nishikata, T.; Abela, A. R.; Lipshutz, B. H. Angew. Chem. Int. Ed. 2010, 49, 781; (g) Arockiam, P. B.; Fischmeister, C.; Bruneau, C.; Dixneuf, P. H. Angew. Chem. Int. Ed. 2010, 49, 6629; (h) Joucla, L.; Batail, N.; Djakovitch, L. Adv. Synth. Catal. 2010, 352, 2929; (i) Ackermann, L.; Pospech, J. Org. Lett. 2011, 13, 4153; (j) Chen, F.; Min, Q. Q.; Zhang, X. G. J. Org. Chem. 2012, 77, 2992; (k) Su, Y.-X.; Deng, Y.-H.; Ma, T.-T.; Li, Y.-Y.; Sun, L.-P. Green Chem. 2012, 14, 1979; (l) Ackermann, L.; Pospech, J.; Potukuchi, H. K. Org. Lett. 2012, 14, 2146; (m) Arockiam, P. B.; Fischmeister, C.; Bruneau, C.; Dixneuf, P. H. Green Chem. 2013, 15, 67; (n) Rao, H. H.; Ma, X. Y.; Liu, Q. Z.; Li, Z. F.; Cao, S. L.; Li, C. J. Adv. Synth. Catal. 2013, 355, 2191; (o) Islam, S.; Larrosa, I. Chem. Eur. J. 2013, 19, 15093.
[13] Wang, B.; Nack, W. A.; He, G.; Zhang, S. Y.; Chen, G. Chem. Sci. 2014, 5, 3952.
[14] (a) Wu, Z.; Luo, F.; Chen, S.; Li, Z.; Xiang, H.; Zhou, X. Chem. Commun. 2013, 49, 7653; (b) Wu, Z.; Chen, S.; Hu, C.; Li, Z.; Xiang, H.; Zhou, X. ChemCatChem 2013, 5, 2839.
/
| 〈 |
|
〉 |