

Visible Light Promoted Benzylic Csp3-H Bond Activation and Functionalization
Received date: 2016-08-16
Revised date: 2016-09-20
Online published: 2016-09-27
Supported by
Project supported by the 973 Program (2012CB725302), the National Natural Science Foundation of China (21390400, 21520102003, 21272180 and 21302148), and the Research Fund for the Doctoral Program of Higher Education of China (20120141130002) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1030) and the Ministry of Science and Technology of China (2012YQ120060), the Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated.
In recent years, visible-light-promoted photoredox catalytic activation of organic molecules has been flourishing vigorously. This kind of methodology usually takes advantage of transition-metal complexes and organic dyes as photosensitizers, which can directly react with organic substrates through a single-electron-transfer (SET) progress under visible light irradiation. It's operable to construct C-X (X=C, N, O …) bond via the radical or radical ion generated during the SET process. On the basis of different key intermediates, this highlight gives a brief summary on the recent development of visible light promoted benzylic Csp3-H activation and functionalization.
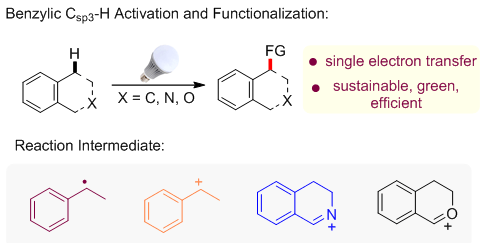
Pei Pengkun , Zhang Fan , Yi Hong , Lei Aiwen . Visible Light Promoted Benzylic Csp3-H Bond Activation and Functionalization[J]. Acta Chimica Sinica, 2017 , 75(1) : 15 -21 . DOI: 10.6023/A16080417
[1] (a) Vanjari, R.; Singh, K. N. Chem. Soc. Rev. 2015, 44, 8062;
(b) Zhang, J.; Lu, Q.-Q.; Liu, C.; Lei, A.-W. Chin. J. Org. Chem. 2015, 35(4), 743. (张剑, 陆庆全, 刘超, 雷爱文, 有机化学, 2015, 35(4), 743.);
(c) Huang, Z.; Tang, S.; Lei, A. Sci. Bull. 2015, 60, 1391.
[2] Prier, C. K.; Rankic, D. A.; MacMillan, D. W. Chem. Rev. 2013, 113, 5322.
[3] Koike, T.; Akita, M. Inorg. Chem. Front. 2014, 1, 562.
[4] Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850.
[5] (a) Xi, Y.; Yi, H.; Lei, A.-W. Org. Biomol. Chem. 2013, 11, 2387;
(b) Zeng, T.-T.; Xuan, J.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Imaging Science and Photochemistry 2014, 32, 415. (曾婷婷, 宣俊, 陈加荣, 陆良秋, 肖文精, 影像科学与光化学, 2014, 32, 415.)
[6] Hopkinson, M. N.; Sahoo, B.; Li, J.-L.; Glorius, F. Chem. Eur. J. 2014, 20, 3874.
[7] Nicewicz, D. A.; Nguyen, T. M. ACS Catal. 2014, 4, 355.
[8] Hari, D. P.; Konig, B. Angew. Chem. Int. Ed. 2013, 52, 4734.
[9] Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527.
[10] Xuan, J.; Xiao, W.-J. Angew. Chem. Int. Ed. 2012, 51, 6828.
[11] Xia, J.-B.; Zhu, C.; Chen, C. J. Am. Chem. Soc. 2013, 135, 17494.
[12] Xia, J.-B.; Zhu, C.; Chen, C. Chem. Commun. 2014, 50, 11701.
[13] Qvortrup, K.; Rankic, D. A.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 626.
[14] Hager, D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 16986.
[15] Rabet, P. T. G.; Fumagalli, G.; Boyd, S.; Greaney, M. F. Org. Lett. 2016, 18, 1646.
[16] Hou, T.-Y.; Lu, P.; Li, P.-X. Tetrahedron Lett. 2016, 57, 2273.
[17] Lu, P.; Hou, T.-Y.; Gu, X.-Y.; Li, P.-X. Org. Lett. 2015, 17, 1954.
[18] Pandey, G.; Laha, R.; Singh, D. J. Org. Chem. 2016, 81, 7161.
[19] Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Angew. Chem. Int. Ed. 2011, 50, 7171.
[20] Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem. Eur. J. 2012, 18, 620.
[21] Zhong, J.-J.; Meng, Q.-Y.; Wang, G.-X.; Liu, Q.; Chen, B.; Feng, K.; Tung, C.-H.; Wu, L.-Z. Chem. Eur. J. 2013, 19, 6443.
[22] Gao, X.-W.; Meng, Q.-Y.; Li, J.-X.; Zhong, J.-J.; Lei, T.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. ACS Catal. 2015, 2391.
[23] Zheng, Y. W.; Chen, B.; Ye, P.; Feng, K.; Wang, W.; Meng, Q. Y.; Wu, L.-Z.; Tung, C.-H. J. Am. Chem. Soc. 2016, 138, 10080.
[24] Zhong, J.-J.; Meng, Q.-Y.; Liu, B.; Li, X.-B.; Gao, X.-W.; Lei, T.; Wu, C.-J.; Li, Z.-J.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2014, 16, 1988.
[25] Zhong, J.-J.; Wu, C.-J.; Meng, Q.-Y.; Gao, X.-W.; Lei, T.; Tung, C.-H.; Wu, L.-Z. Adv. Synth. Catal. 2014, 356, 2846.
[26] Meng, Q.-Y.; Zhong, J.-J.; Liu, Q.; Gao, X.-W.; Zhang, H.-H.; Lei, T.; Li, Z.-J.; Feng, K.; Chen, B.; Tung, C.-H.; Wu, L.-Z. J. Am. Chem. Soc. 2013, 135, 19052.
[27] Ye, P.; Wang, D.-H.; Chen, B.; Meng, Q.-Y.; Tung, C.-H.; Wu, L.-Z. Sci. China. Chem. 2016, 59, 175.
[28] Xiang, M.; Meng, Q.-Y.; Li, J.-X.; Zheng, Y.-W.; Ye, C.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem. Eur. J. 2015, 21, 18080.
[29] Moriyama, K.; Takemura, M.; Togo, H. Org. Lett. 2012, 14, 2414.
[30] Xu, J.; Luo, L.; Xiao, G.; Zhang, Z.; Lin, H.; Wang, X.; Long, J. ACS Catal. 2014, 4, 3302.
[31] Muhldorf, B.; Wolf, R. Chem. Commun. 2015, 51, 8425.
[32] Neu, H. M.; Jung, J.; Baglia, R. A.; Siegler, M. A.; Ohkubo, K.; Fukuzumi, S.; Goldberg, D. P. J. Am. Chem. Soc. 2015, 137, 4614.
[33] Yi, H.; Bian, C.; Hu, X.; Niu, L.; Lei, A. Chem. Commun. 2015, 51, 14046.
/
| 〈 |
|
〉 |