

A New Fluorometric Turn-on Assay for Carboxylesterase and Inhibitor Screening Based on Aggregation Induced Emission Behavior of Tetraphenylethylene Molecules
Received date: 2016-08-15
Online published: 2016-10-10
Supported by
Project supported by the 973 Program (Nos. 2013CB733700, 2013CB834700) and Beijing Natural Science Foundation (No. 2162048).
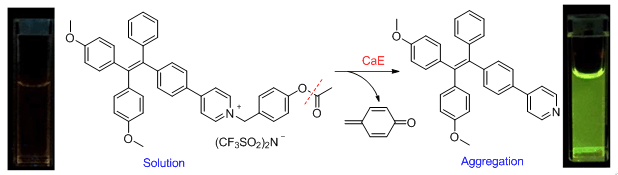
It is known that carboxylesterase (CaE) are a group of isoenzymes commonly distributed in mammalian organs, and they can catalyze the hydrolysis of carboxyl ester. As a result, they play an important role in detoxification of narcotics or chemical toxin clearance. Moreover, they serve as important drug candidates for protein-based therapeutics or drug targets for chemotherapeutic prodrug activation. It is reported recently that human plasma carboxylesterase can be a novel biomarker candidate for hepatocellular carcinoma. Therefore, establishing a reliable fluorescent system for detecting carboxylesterase is of great importance in terms of biochemical studies as well as clinical applications. Herein, we report a new fluorometric turn-on assay for carboxylesterase activity and inhibitor screening with compound 1 by utilizing the aggregation-induced emission (AIE) feature of tetraphenylethylene (TPE) molecules. The sensing mechanism is illustrated in Figure 1 and explains as follows:(i) the pyridinium moiety may render compound 1 water-soluble. As a result it is anticipated that compound 1 is weakly emissive in aqueous solutions according to previous studies; (ii) the incubation of carboxylesterase with compound 1 can result in cleaving the carboxylic ester bond, followed by hydrolysis and 1,6-elimination of p-quinonemethide to yield the p-pyridine substituted TPE (TPE-Py). TPE-Py is not soluble in aqueous solutions, thus aggregation will occur and turn on the fluorescence of TPE moiety based on the AIE feature of TPE compounds. In this way, compound 1 can be employed for the fluorescence turn-on assay for carboxylesterase activity. The results reveal that the buffer solution of compound 1 emitted very weakly. However, the green fluorescence emission was switched on after addition of carboxylesterase. Carboxylesterase at concentrations as low as 5.67×10-5 U/mL can be assayed with compound 1. Further results clearly indicate that compound 1 can be utilized not only for carboxylesterase activity assay but also for the corresponding inhibitor screening. More importantly, this probe can be applied for detection of carboxylesterases in living cells.
Yang Yang , Huang Yanyan , Zhang Guanxin , Zhao Rui , Zhang Deqing . A New Fluorometric Turn-on Assay for Carboxylesterase and Inhibitor Screening Based on Aggregation Induced Emission Behavior of Tetraphenylethylene Molecules[J]. Acta Chimica Sinica, 2016 , 74(11) : 871 -876 . DOI: 10.6023/A16080415
[1] (a) Satoh, T.; Hosokawa, M. Chem. Biol. Interact. 2006, 162, 195;
(b) Jokanovi?, M. Toxicol. Lett. 2009, 188, 1;
(c) Hosokawa, M. Molecules 2008, 13, 412;
(d) Nagashima, S.; Yagyu, H.; Takahashi, N.; Takahashi, M.; Tsuchita, T.; Tazoe, F.; Wang, X.; Bayasgalan, T.; Sato, N.; Okada, K.; Nagasaka, S.; Gotoh, T.; Kojima, M.; Hyodo, M.; Horie, H.; Hosoya, Y.; Okada, M.; Yasuda, Y.; Fujiwara, H.; Ohwada, M.; Iwamoto, S.; Suzuki, M.; Nagai, H.; Ishibashi, S. J. Atheroscler. Thromb. 2011, 18, 190.
[2] (a) Na, K.; Lee, E.; Lee, H.; Kim, K.; Lee, H.; Jeong, S.; Jeong, A.; Cho, S.; Kim, S.; Song, S.; Kim, K.; Cho, S.; Kim H.; Paik, Y. Proteomics 2009, 9, 3989;
(b) Yamada, S.; Richardson, K.; Tang, M.; Halaschek-Wiener, J.; Cook, V.; FitzGerald, J.; Elwood, K.; Marra, F.; Brooks-Wilson, A. Pharmacogenomics J. 2010, 10, 524.
[3] (a) Yue, Q.; Yang, H.; Li, D.; Wang, J. Anim. Feed Sci. Technol. 2009, 153, 169;
(b) Koitka, M.; Höechel, J.; Obst, D.; Rottman, A.; Gieschen, H.; Borchert, H. Anal. Biochem. 2008, 381, 113.
[4] Koo, Y.; Yoon, E.; Song, J.; Seo, H.; Kim, J.; Lee, Y.; Lee, J.; Cheong, J.; Choi, Y. J. Chromatogr. B 2008, 863, 80.
[5] Steinkamp, T.; Schweppe, F.; Krebs, B.; Karst, U. Analyst 2003, 128, 29.
[6] (a) Zhang, Y.; Chen, W.; Feng, D.; Shi, W.; Li, X.; Ma, H. Analyst 2012, 137, 716;
(b) Wu, Y.; Huang, S.; Zeng, F.; Wang, J.; Yu, C.; Huang, J.; Xie, H.; Wu, S. Chem. Commun. 2015, 51, 12791;
(c) Gao, M.; Hu, Q.; Feng, G.; Tang, B.; Liu, B. J. Mater. Chem. B 2014, 2, 3438;
(d) Jin, Q.; Feng, L.; Wang, D.; Dai, Z.; Wang, P.; Zou, L.; Liu, Z.; Wang, J.; Yu, Y.; Ge, G.; Cui, J.; Yang, L. ACS Appl. Mater. Interfaces 2015, 7, 28474.
[7] (a) Sinkeldam, R.; Greco, N.; Tor, Y. Chem. Rev. 2010, 110, 2579;
(b) Zhang, X.; Yin, J.; Yoon, J. Chem. Rev. 2014, 114, 4918;
(c) Carter, K.; Young, A.; Palmer, A. Chem. Rev. 2014, 114, 4564;
(d) Li, X.; Gao, X.; Shi, W.; Ma, H. Chem. Rev. 2014, 114, 590;
(e) Du, J.; Hu, M.; Fan, J.; Peng, X. Chem. Soc. Rev. 2012, 41, 4511;
(f) Kim, H.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Chem. Soc. Rev. 2011, 40, 79;
(g) Huang, X.; Zhang, G.; Zhang, D. Acta Chim. Sinica 2012, 70, 2133. (黄显虹, 张关心, 张德清, 化学学报, 2012, 70, 2133.)
[8] Luo, J.; Xie, Z.; Lam, J.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Chem. Commun. 2001, 18, 1740.
[9] Hong, Y.; Lam, J.; Tang, B. Chem. Commun. 2009, 29, 4332.
[10] (a) Hong, Y.; Lam, J.; Tang, B. Chem. Soc. Rev. 2011, 40, 5361;
(b) Mei, J.; Leung, N.; Kwok, R.; Lam, J.; Tang, B. Chem. Rev. 2015, 115, 11718.
[11] (a) Liang, J.; Tang, B.; Liu, B. Chem. Soc. Rev. 2015, 44, 2798.
(b) Lu, H.; Xu, B.; Dong, Y.; Chen, F.; Li, Y.; Li, Z.; He, J.; Li, H.; Tian, W. Langmuir 2010, 26, 6838.
(c) Tong, H.; Hong, Y.; Dong, Y.; Haussler, M.; Li, Z.; Lam, J.; Dong, Y.; Sung, H.; Williams, I.; Tang, B. J. Phys. Chem. B 2007, 111, 11817.
(d) Shen, X.; Huang, G.; Li, K.; Zhang, G.; Zhang, D. Sci. China Chem. 2013, 56, 1197.
(e) Qian, L.; Tong, B.; Zhi, J.; Yang, F.; Shen, J.; Shi, J.; Dong, Y. Acta Chim. Sinica 2008, 66, 1134(钱立军, 佟斌, 支俊格, 杨帆, 申进波, 石建兵, 董宇平, 化学学报, 2008, 66, 1134.);
(f) Li, Q.; Li, Z. Sci. China Chem. 2015, 58, 1800.
[12] (a) Qi, Q.; Qian, J.; Ma, S.; Xu, B.; Zhang, S.; Tian, W. Chem. Eur. J. 2015, 21, 1149;
(b) Chen, M.; Li, L.; Nie, H.; Tong, J.; Yan, L.; Xu, B.; Sun, J.; Tian, W.; Zhao, Z.; Qin, A.; Tang, B. Chem. Sci. 2015, 6, 1932;
(c) Ruan, Z.; Li, C.; Li, J.; Qin, J.; Li, Z. Sci. Rep. 2015, 5, 15987;
(d) Huang, Y.; Zhang, P.; Gao, M.; Zeng, F.; Qin, A.; Wu, S.; Tang, B. Chem. Commun. 2016, 52, 7288;
(e) Xun, Z. Q.; Tang, H.; Zeng, Y.; Chen, J.; Yu, T.; Zhang, X.; Li, Y. Acta Chim. Sinica 2015, 73, 819. (寻知庆, 唐海云, 曾毅, 陈金平, 于天君, 张小, 李嫕, 化学学报, 2015, 73, 819.)
(f) Sun, J.; Zhang, G.; Jia, X.; Xue, P.; Jia, J.; Lu, R. Acta Chim. Sinica 2016, 74, 165. (孙静波, 张恭贺, 贾小宇, 薛鹏冲, 贾俊辉, 卢然, 化学学报, 2016, 74, 165.);
(g) Xia, Z.; Shao, A.; Li, Q.; Zhu, S.; Zhu, W. Acta Chim. Sinica 2016, 74, 351(夏志清, 邵安东, 李强, 朱世琴, 朱为宏, 化学学报, 2016, 74, 351).
[13] (a) Wang, M.; Zhang, G.; Zhang, D.; Zhu, D.; Tang, B. J. Mater. Chem. 2010, 20, 1858;
(b) Zhang, G.; Hu, F.; Zhang, D. Langmuir 2015, 31, 4593.
[14] (a) Sun, F.; Zhang, G.; Zhang, D.; Xue, L.; Jiang, H.; Zhang, D. Org. Lett. 2011, 13, 6378;
(b) Liu, L.; Zhang, G.; Xiang, J.; Zhang, D.; Zhu, D. Org. Lett. 2008, 10, 4581;
(c) Gui, S.; Huang, Y.; Hu, F.; Jin, Y.; Zhang, G.; Yan, L.; Zhang, D.; Zhao, R. Anal. Chem. 2015, 87, 1470;
(d) Huang, X.; Gu, X.; Zhang, G.; Zhang, D. Chem. Commun. 2012, 48, 12195.
[15] (a) Wang, M.; Zhang, D.; Zhang, G.; Zhu, D. Chem. Commun. 2008, 4469;
(b) Wang, M.; Zhang, D.; Zhang, G.; Tang, Y.; Wang, S.; Zhu, D. Anal. Chem. 2008, 80, 6443;
(c) Wang, M.; Gu, X.; Zhang, G.; Zhang, D.; Zhu, D. Anal. Chem. 2009, 81, 4444.
[16] (a) Peng, L.; Zhang, G.; Zhang, D.; Xiang, J.; Zhao, R.; Wang, Y.; Zhu, D. Org. Lett. 2009, 11, 4014;
(b) Shen, X.; Zhang, G.; Zhang, D. Org. Lett. 2012, 14, 1744;
(c) Peng, L.; Wang, M.; Zhang, G.; Zhang, D.; Zhu, D. Org. Lett. 2009, 11, 1943;
(d) Dong, X.; Hu, F.; Liu, Z.; Zhang, G.; Zhang, D. Chem. Commun. 2015, 51, 3892.
[17] (a) Hu, F.; Huang, Y.; Zhang, G.; Zhao, R.; Yang, H.; Zhang, D. Anal. Chem. 2014, 86, 7987;
(b) Huang, Y.; Hu, F.; Zhao, R.; Zhang, G.; Yang, H.; Zhang, D. Chem. Eur. J. 2014, 20, 158;
(c) Chen, W.; Li, Q.; Zheng, W.; Hu, F.; Zhang, G.; Wang, Z.; Zhang, D.; Jiang, X. Angew. Chem., Int. Ed. 2014, 53, 13734;
(d) Huang, Y.; Zhang, G.; Hu, F.; Jin, Y.; Zhao, R.; Zhang, D. Chem. Sci. 2016, DOI:10.1039/c6sc02395a.
/
| 〈 |
|
〉 |