

Germanium Nanotube as the Catalyst for Oxygen Reduction Reaction: Performance and Mechanism
Received date: 2016-08-29
Revised date: 2016-10-09
Online published: 2016-10-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51602270, 51671004).
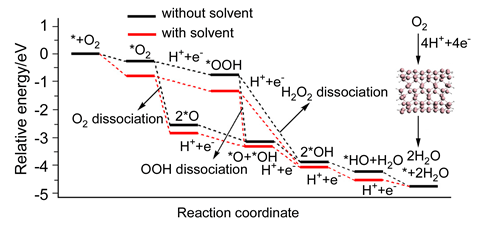
One of the major technical barriers to the commercialization of proton exchange membrane fuel cells is the high cost of Pt-based oxygen reduction reaction (ORR) electrocatalysts. In this paper, the ORR catalytic performance and the possible mechanism on (5,5) germanium nanotube (GeNT) were studied by density functional theory methods using DZP basis set. The results indicate that the ORR on the GeNT may undergo three mechanisms including O2 dissociation, OOH dissociation and H2O2 dissociation. For any of the above mechanism, the whole process could easily take place on the GeNT with a complete 4e- ORR pathway. The adsorption properties of the ORR intermediates, especially for O and OH, are also very important for evaluating the catalytic performance. The calculated adsorption energies of the above species are -4.33 and -2.21 eV respectively, much close to those on the Pt. Furthermore, the adsorption energy of H2O on the GeNT is only -0.05 eV, much weaker than the O2 binding, indicating the catalytic cycle of ORR could repeat most easily on the GeNT. Therefore, both the reaction energies of the ORR steps and the adsorption energies of ORR intermediates show that the current GeNT model has the catalytic performance similar to that of precious Pt catalyst. Furthermore, the solvent effect was also studied by using three-water-molecule clusters as the real solvent. The obtained results indicate that the solvent effect could affect the geometrical structure of some adsorbed ORR intermediates, such as atomic O. This would lead to the decrease of the heat loss during the O2 dissociation mechanism. The decreased heat loss would accelerate the following electron transfer steps, due to the fact that an effective electrocatalyst must make the energy loss as small as possible for non-electron-transfer step, in which case the cathode electrocatalyst would deliver all the Gibbs energy of the ORR as electrical work. With solvation, the heat loss is slightly increased from *O2 to *OOH, and decreased from *OOH to *OH in the H2O2 dissociation mechanism, which are also more favorable for ORR.
Chen Xin , Yan Huijun , Xia Dingguo . Germanium Nanotube as the Catalyst for Oxygen Reduction Reaction: Performance and Mechanism[J]. Acta Chimica Sinica, 2017 , 75(2) : 189 -192 . DOI: 10.6023/A16080451
[1] Peng, S.; Xu, X.; Zhang, J.; Liu, Y.; Lu, S.; Xiang, Y. Acta Chim. Sinica 2015, 73, 137. (彭思侃, 徐鑫, 张劲, 刘祎阳, 卢善富, 相艳, 化学学报, 2015, 73, 137.)
[2] Xu, X.; Peng, S.; Zhang, J.; Lu, S.; Xiang, Y. Acta Chim. Sinica 2016, 74, 271. (徐鑫, 彭思侃, 张劲, 卢善富, 相艳, 化学学报, 2016, 74, 271.)
[3] Cheng, F.; Chen, J. Chem. Soc. Rev. 2012, 41, 2172.
[4] Cao, R.; Lee, J. S.; Liu, M.; Cho, J. Adv. Energy Mater. 2012, 2, 816.
[5] Bashyam, R.; Zelenay, P. Nature 2006, 443, 63.
[6] Wu, G.; More, K. L.; Johnston, C. M.; Zelenay, P. Science 2011, 332, 443.
[7] Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J. P. Science 2009, 324, 71.
[8] Kattel, S.; Wang, G. J. Phys. Chem. Lett. 2014, 5, 452.
[9] An, L.; Huang, W.; Zhang, N.; Chen, X.; Xia, D. J. Mater. Chem. A 2014, 2, 62.
[10] Cao, B.; Veith, G. M.; Diaz, R. E.; Liu, J.; Stach, E. A.; Adzic, R. R.; Khalifah, P. G. Angew. Chem., Int. Ed. 2013, 52, 10753.
[11] Chen, X.; Qiao, Q.; An, L.; Xia, D. J. Phys. Chem. C 2015, 119, 11493.
[12] Chen, X.; Chen, S.; Wang, J. Appl. Surf. Sci. 2016, 379, 291.
[13] Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. J. Am. Chem. Soc. 2014, 136, 4394.
[14] Nayak, S.; Biedermann, P. U.; Stratmann, M.; Erbe, A. Phys. Chem. Chem. Phys. 2013, 15, 5771.
[15] Nayak, S.; Biedermann, P. U.; Stratmann, M.; Erbe, A. Electrochim. Acta 2013, 106, 472.
[16] Seifert, G.; Köhler, T.; Hajnal, Z.; Frauenheim, T. Solid State Commun. 2001, 119, 653.
[17] Alam, K. M.; Ray, A. K. Nanotechnology 2007, 18, 495706.
[18] Rathi, S. J.; Ray, A. K. Nanotechnology 2008, 19, 335706.
[19] Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jonsson, H. J. Phys. Chem. B 2004, 108, 17886.
[20] Greeley, J.; Stephens, I. E. L.; Bondarenko, A. S.; Johansson, T. P.; Hansen, H. A.; Jaramillo, T. F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J. K. Nat. Chem. 2009, 1, 552.
[21] Lee, K. R.; Jung, Y.; Woo, S. I. ACS Comb. Sci. 2012, 14, 10.
[22] Chen, R.; Li, H.; Chu, D.; Wang, G. J. Phys. Chem. C 2009, 113, 20689.
[23] Sha, Y.; Yu, T. H.; Liu, Y.; Merinov, B. V.; Goddard, W. A. J. Phys. Chem. Lett. 2010, 1, 856.
[24] Han, B. C.; Miranda, C. R.; Ceder, G. Phys. Rev. B 2008, 77, 075410.
[25] Anderson, A. B. Phys. Chem. Chem. Phys. 2012, 14, 1330.
[26] te Velde, G.; Bickelhaupt, F. M.; Baerends, E. J.; Fonseca Guerra, C.; van Gisbergen, S. J. A.; Snijders, J. G.; Ziegler, T. J. Comput. Chem. 2001, 22, 931.
[27] Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
[28] Zhang, L.; Xia, Z. J. Phys. Chem. C 2011, 115, 11170.
[29] del Cueto, M.; Ocon, P.; Poyato, J. M. L. J. Phys. Chem. C 2015, 119, 2004.
[30] Ou, L.; Yang, F.; Liu, Y.; Chen, S. J. Phys. Chem. C 2009, 113, 20657.
[31] Roudgar, A.; Eikerling, M.; van Santen, R. Phys. Chem. Chem. Phys. 2010, 12, 614.
[32] Fang, Y. H.; Liu, Z. P. J. Phys. Chem. C 2009, 113, 9765.
[33] Hamada, I.; Morikawa, Y. J. Phys. Chem. C 2008, 112, 10889.
/
| 〈 |
|
〉 |