

Fluoranthene-Modified Tetraphenylethene Derivatives: Synthesis, Aggregation-Enhanced Emission Characteristic and Their Highly Sensitive Detection of Picric Acid
Received date: 2016-08-14
Online published: 2016-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 51273175), and National Basic Research Program of China (973 Program, No. 2012CB834704).
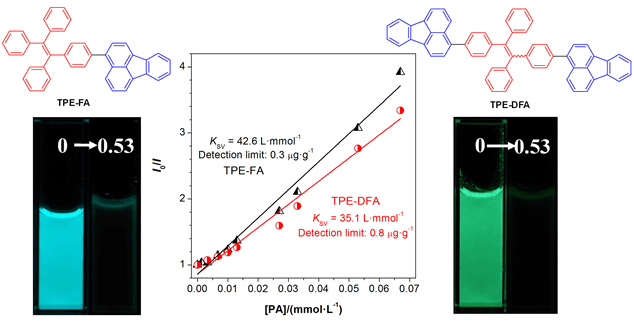
Aggregation-induced emission (AIE) active compounds and materials have become one of the hottest research topics worldwide, due to their unprecedented merits such as ultra-high fluorescence quantum efficiencies as aggregates or in solid state. Up to now, it is still extremely crucial and fundamental to expand the AIE-genic molecular systems for this research area. Here, we prepared two fluoranthene-modified tetraphenylethene (TPE) derivatives, TPE-FA and TPE-DFA, through the Suzuki-Miyaura coupling between boronate-bearing TPE and bromo-bearing fluoranthene under mild reaction condition. The conjugation between fluoranthene and TPE moieties assures that the as-prepared TPE-FA and TPE-DFA both possess aggrega-tion-enhanced emission (AEE) characteristics. The emission maximum of TPE-FA and TPE-DFA as aggregates in THF/water mixtures is at 477 nm and 494 nm, and the absolute quantum yields of the two compounds in solid films are as high as 74.1% and 40.4%, respectively. They can be utilized as fluorescent probes for picric acid with high sensitivity. Their quenching coef-ficients can be as high as 4×104 L·mol-1, while their detection limits can be lower than 1 μg·g-1. These AEE-active molecules are promising to act as fluorescent probes in the detection of other nitro-substituted electron-deficient molecules.
Bai Wei , Shi Yang , Song Chen , He Jie , Qin Anjun , Sun Jing Zhi , Tang Ben Zhong . Fluoranthene-Modified Tetraphenylethene Derivatives: Synthesis, Aggregation-Enhanced Emission Characteristic and Their Highly Sensitive Detection of Picric Acid[J]. Acta Chimica Sinica, 2016 , 74(11) : 893 -901 . DOI: 10.6023/A16080410
[1] (a) Basabe-Desmonts, L.; Reinhoudt, D. N.; Crego-Calama, M. Chem. Soc. Rev. 2007, 36, 993;
(b) Bao, Y.; Wang, T.; Li, Q.; Du, F.; Bai, R.; Smet, M.; Dehaen, W. Polym. Chem. 2014, 5, 792;
(c) Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Chem. Soc. Rev. 2013, 42, 622;
(d) Dias, F. B.; Bourdakos, K. N.; Jankus, V.; Moss, K. C.; Kamtekar, K. T.; Bhalla, V.; Santos, J.; Bryce, M. R.; Monkman, A. P. Adv. Mater. 2013, 25, 3707;
(e) Li, W.; Pan, Y.; Xiao, R.; Peng, Q.; Zhang, S.; Ma, D.; Li, F.; Shen, F.; Wang, Y.; Yang, B.; Ma, Y. Adv. Funct. Mater. 2014, 24, 1609;
(f) Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S. Y.; Yasuda, T.; Adachi, C. Adv. Mater. 2015, 27, 2096.
[2] Birks, J. B. Photophysics of Aromatic Molecules, Wiley, London, 1970.
[3] (a) Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 37, 1740;
(b) Mei, J.; Hong, Y.; Lam, J. W. Y.; Qin, A.; Tang, Y.; Tang, B. Z. Adv. Mater. 2014, 26, 5429;
(c) Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam, J. W. Y.; Tang, B. Z. Chem. Rev. 2015, 115, 11718;
(d) Wang, H.; Zhao, E.; Lam, J. W. Y.; Tang, B. Z. Mater. Today 2015, 18, 365.
[4] (a) Wang, M.; Zhang, G.; Zhang, D.; Zhu, D.; Tang, B. Z. J. Mater. Chem. 2010, 20, 1858;
(b) Liang, J.; Tang, B. Z.; Liu, B. Chem. Soc. Rev. 2015, 44, 2798;
(c) Zhang, X.; Wang, K.; Liu, M.; Zhang, X.; Tao, L.; Chen, Y.; Wei, Y. Nanoscale 2015, 7, 11486;
(d) Dong, Y. Q.; Lam, J. W. Y.; Tang, B. Z. J. Phys. Chem. Lett. 2015, 6, 3429;
(e) Yang, J.; Huang, J.; Li, Q.; Li, Z. J. Mater. Chem. C 2016, 4, 2663.
[5] (a) Tseng, N.-W.; Liu, J.; Ng, J. C. Y.; Lam, J. W. Y.; Sung, H. H. Y.; Williams, I. D.; Tang, B. Z. Chem. Sci. 2012, 3, 493;
(b) Liang, G.; Lam, J. W. Y.; Qin, W.; Li, J.; Xie, N.; Tang, B. Z. Chem. Commun. 2014, 50, 1725;
(c) Parrot, E. P. J.; Tan, N. Y.; Hu, R.; Zeitler, J. A.; Tang, B. Z.; Pickwell-MacPherson, E. Mater. Horiz. 2014, 1, 251;
(d) Yuan, H.; Wang, K.; Yang, K.; Liu, B.; Zou, B. J. Phys. Chem. Lett. 2014, 5, 2968;
(e) Sun, G.; Zhao, Y.; Liang, W. J. Chem. Theory Comput. 2015, 11, 2257.
[6] (a) Zhao, Z.; Lam, J. W. Y.; Tang, B. Z. J. Mater. Chem. 2012, 22, 23726;
(b) Wang, J.; Mei, J.; Hu, R.; Sun, J. Z.; Qin, A.; Tang, B. Z. J. Am. Chem. Soc. 2012, 134, 9956;
(c) Bai, W.; Wang, Z.; Tong, J.; Mei, J.; Qin, A.; Sun, J. Z.; Tang, B. Z. Chem. Commun. 2015, 51, 1089;
(d) Chen, S.; Hong, Y.; Zeng, Y.; Sun, Q.; Liu, Y.; Zhao, E.; Bai, G.; Qu, J.; Hao, J.; Tang, B. Z. Chem. Eur. J. 2015, 21, 4315;
(e) Wang, C.; Xu, B.; Li, M.; Chi, Z.; Xie, Y.; Li, Q.; Li, Z. Mater. Horiz. 2016, 3, 220.
[7] (a) He, J.; Xu, B.; Chen, F.; Xia, H.; Li, K.; Ye, L.; Tian, W. J. Phys. Chem. C 2009, 113, 9892;
(b) Lu, H.; Xu, B.; Dong, Y.; Chen, F.; Li, Y.; Li, Z.; He, J.; Li, H.; Tian, W. Langmuir 2010, 26, 6838;
(c) Chen, J. L.; Ma, S. Q.; Xu, B.; Zhang, J. B.; Dong, Y. J.; Tian, W. J. Chin. Sci. Bull. 2013, 58, 2747.
[8] (a) Chen, M.; Li, L.; Nie, H.; Tong, J.; Yan, L.; Xu, B.; Sun, J. Z.; Tian, W.; Zhao, Z.; Qin, A.; Tang, B. Z. Chem. Sci. 2015, 6, 1932;
(b) Chen, M.; Li, L.; Nie, H.; Shi, Y.; Mei, J.; Wang, J.; Sun, J. Z.; Qin, A.; Tang, B. Z. Chem. Commun. 2015, 51, 10710;
(c) Pan, L. X.; Luo, W. W.; Chen, M.; Liu, J. K.; Xu, L.; Hu, R. R.; Zhao, Z. J.; Qin, A. J.; Tang, B. Z. Chin. J. Org. Chem. 2016, 36, 1316(in Chinese). (潘凌翔, 罗文文, 陈明, 刘峻恺, 徐露, 胡蓉蓉, 赵祖金, 秦安军, 唐本忠, 有机化学, 2016, 36, 1316.)
[9] (a) Hu, R.; Lam, J. W. Y.; Liu, Y.; Zhang, X.; Tang, B. Z. Chem. Eur. J. 2013, 19, 5617;
(b) Li, L.; Chen, M.; Zhang, H.; Nie, H.; Sun, J. Z.; Qin, A.; Tang, B. Z. Chem. Commun. 2015, 51, 4830;
(c) Xun, Z. Q.; Tang, H. Y.; Zeng, Y.; Chen, J. P.; Yu, T. J.; Zhang, X. H.; Li, Y. Acta Chim. Sinica 2015, 73, 819(in Chinese). (寻知庆, 唐海云, 曾毅, 陈金平, 于天君, 张小辉, 李嫕, 化学学报, 2015, 73, 819.)
[10] Wang, Z.; Bai, W.; Tong, J.; Wang, Y. J.; Qin, A.; Sun, J. Z.; Tang, B. Z. Chem. Commun. 2016, 52, 10365.
[11] Yang, J.; Li, L.; Yu, Y.; Ren, Z.; Peng, Q.; Ye, S.; Li, Q.; Li, Z. Mater. Chem. Front. 2017, DOI:10.1039/c6qm00014b.
[12] (a) Song, Z.; Zhang, W.; Jiang, M.; Sung, H. H. Y.; Kwok, R. T. K.; Nie, H.; Williams, I. D.; Liu, B.; Tang, B. Z. Adv. Funct. Mater. 2016, 26, 824;
(b) Zhao, Z.; Chen, S.; Chan, C. Y. K.; Lam, J. W. Y.; Jim, C. K. W.; Lu, P.; Chang, Z.; Kwok, H. S.; Qiu, H.; Tang, B. Z. Chem. Asian J. 2012, 7, 484;
(c) Tong, J.; Wang, Y.; Mei, J.; Wang, J.; Qin, A.; Sun, J. Z.; Tang, B. Z. Chem. Eur. J. 2014, 20, 4661;
(d) Wang, Y. J.; Shi, Y.; Wang, Z.; Zhu, Z.; Zhao, X.; Nie, H.; Qian, J.; Qin, A.; Sun, J. Z.; Tang, B. Z. Chem. Eur. J. 2016, 22, 9784.
[13] (a) Shao, A.; Xie, Y.; Zhu S.; Guo, Z.; Zhu, S.; Guo, J.; Shi, P.; James, T. D.; Tian, H.; Zhu, W.-H. Angew. Chem., Int. Ed. 2015, 54, 7275;
(b) Guo, Z.; Shao, A.; Zhu, W.-H. J. Mater. Chem. C 2016, 4, 2640;
(c) Xia, Z. Q.; Shao, A. D.; Li, Q.; Zhu, S. Q.; Zhu, W. H. Acta Chim. Sinica 2016, 74, 351(in Chinese). (夏志清, 邵安东, 李强, 朱世琴, 朱为宏, 化学学报, 2016, 74, 351.)
[14] (a) Ding, L.; Ying, H.-Z.; Zhou, Y.; Lei, T.; Pei, J. Org. Lett. 2010, 12, 5522;
(b) Zhou, Y.; Ding, L.; Shi, K.; Dai, Y.-Z.; Ai, N.; Wang, J.; Pei, J. Adv. Mater. 2012, 24, 957;
(c) Zheng, Y.-Q.; Dai, Y.-Z.; Zhou, Y.; Wang, J.-Y.; Pei, J. Chem. Commun. 2014, 50, 1591;
(d) Saranya, G.; Kolandaivel, P.; Senthilkumar, K. J. Phys. Chem. A 2011, 115, 14647;
(e) Goel, A.; Kumar, V.; Chaurasia, S.; Rawat, M.; Prasad, R.; Anand, R. S. J. Org. Chem. 2010, 75, 3656;
(f) Goel, A.; Sharma, A.; Kathuria, M.; Bhattacharjee, A.; Verma, A.; Mishra, P. R.; Nazir, A.; Mitra, K. Org. Lett. 2014, 16, 756;
(g) Han, L.; Zhang, Y.; Chen, W.; Cheng, X.; Ye, K.; Zhang, J.; Wang, Y. Chem. Commun. 2015, 51, 4477.
[15] Li, X.-G.; Liao, Y.; Huang, M.-R.; Strong, V.; Kaner, R. B. Chem. Sci. 2013, 4, 1970.
[16] (a) Venkatramaiah, N.; Kumar, S.; Patil, S. Chem. Eur. J. 2012, 18, 14745;
(b) Kumar, S.; Venkatramaiah, N.; Patil, S. J. Phys. Chem. C 2013, 117, 7236.
[17] Chandrasekaran, Y.; Venkatramaiah, N.; Patil, S. Chem. Eur. J. 2016, 22, 5288.
[18] (a) Feng, H.-T.; Zheng, Y.-S. Chem. Eur. J. 2014, 20, 195;
(b) Wang, X.; Bian, J.; Xu, L.; Wang, H.; Feng, S. Phys. Chem. Chem. Phys. 2015, 17, 32472.
/
| 〈 |
|
〉 |