

Properties of Polymorphism and Acid Response of Pyrrolopyrrole-based Derivative with Aggregation-induced Emission Behavior
Received date: 2016-08-13
Online published: 2016-12-03
Supported by
Project supported by the National Basic Research Program of China (973 Program:2013CB834704) and National Natural Science Foundation of China (Nos.:51328302, 21474009).
A new A-D-A type pyrrolopyrrole-based derivative 4',4"-(2,5-diphenyl-pyrrolo[3,2-b]pyrrole-1,4-diyl)bis ([1,1'-biphenyl]-4-carbonitrile) (DPPDC) was synthesized via Suzuki coupling reaction between 1,4-bis(4-bromophenyl)-2,5-diphenyl-1,4-dihydropyrrolo[3,2-b]pyrrole and 4-cyanophenylboronic acid. The fluorescent emission intensities of DPPDC in pure THF solution and lower fraction of water (φH2O≤60%) mixtures were weak at around 550 nm. When φH2O was 99% in THF/H2O mixtures, the emission was enhanced and blue-shifted at around 505 nm. The maximal fluorescent emission intensity of DPPDC was 11 times higher than that of in pure THF solution, indicating DPPDC exhibiting AIE property. It was also found that four different kinds of crystal structures of DPPDC was cultivated from CHCl2-Hexane, CHCl3-Hexane and CHCl3/Acetone-Hexane systems via solvent slow diffusion method. Four crystals respec-tively emitted blue, azure, green and turquoise at 467, 483, 496 and 493 nm, which manifested the polymorphism-dependent fluorescent emission property of DPPDC. Additionally, trifluoroacetic acid (TFA) could make the emitting color change from yellow to orange-red with as-prepared paper containing DPPDC due to the acid-base interaction. The obvious emitting color change of DPPDC can be used as a visual sensor to detect acid gas.
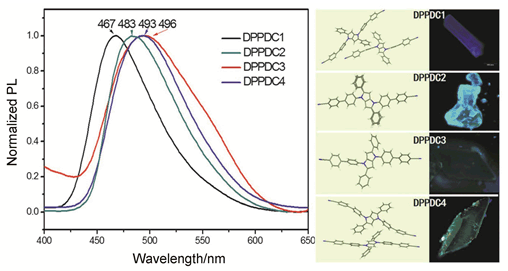
Key words: polymorphism; pyrrolopyrrole; acid response; aggregation-induced emission
Peng Zhe , Ji Yingchun , Wang Zhi , Tong Bin , Shi Jianbing , Dong Yuping . Properties of Polymorphism and Acid Response of Pyrrolopyrrole-based Derivative with Aggregation-induced Emission Behavior[J]. Acta Chimica Sinica, 2016 , 74(11) : 942 -948 . DOI: 10.6023/A16080406
[1] (a) Kim, S.; Kim, B.; Lee, J.; Shin, H.; Park, Y.; Park, J. Mat. Sci. Eng. R 2016, 99, 1;
(b)) Zhao, Z.; Li, Z.; Lam, J. W. Y.; Maldonado, J. L.; Ramos-Ortiz, G.; Liu, Y.; Yuan, W.; Xu, J.; Miao, Q.; Tang, B. Z. Chem. Commun. 2011, 47, 6924;
(c) Fujisawa, K.; Okuda, Y.; Izumi, Y.; Nagamatsu, A.; Rokusha, Y.; Sadaike, Y.; Tsutsumi, O. J. Mater. Chem. C 2014, 2, 3549;
(d) Zhao, N.; Zhang, C.; Lam, J. W. Y.; Zhao, Y. S.; Tang, B. Z. Asian J. Org. Chem. 2014, 3, 118;
(e) Mukherjee, S.; Thilagar, P. J. Mater. Chem. C 2016, 4, 2647;
(f) Pan, L. X.; Luo, W. W.; Chen, M.; Liu, J. K.; Xu, L.; Hu, R. R.; Zhao, Z. J.; Qin, A. J.; Tang, B. Z. Chin. J. Org. Chem. 2016, 36, 1316. (潘凌翔, 罗文文, 陈明, 刘峻恺, 徐露, 胡蓉蓉, 赵祖金, 秦安军, 唐本忠, 有机化学, 2016, 36, 1316.)
[2] Wang, K.; Zhang, H. Y.; Chen, S. Y.; Yang, G. C.; Zhang, J. B.; Tian, W. J.; Su, Z. M.; Wang, Y. Adv. Mater. 2014, 26, 6168.
[3] Dong, Y. J.; Xu, B.; Zhang, J. B.; Tan, X.; Wang, L. J.; Chen, J. L.; Lv, H. G.; Wen, S. P.; Li, B.; Ye, L.; Zou, B.; Tian, W. J. Angew. Chem. Int. Ed. 2012, 51, 10782.
[4] Yoon, S. J.; Chung, J. W.; Gierschner, J.; Kim, K. S.; Choi, M. G.; Kim, D.; Park, S. Y. J. Am. Chem. Soc. 2010, 132, 13675.
[5] Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 1740.
[6] (a) Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B. Sci. Rep. 2013, 3, 1150;
(b) Wang, D.; Qian, J.; Qin, W.; Qin, A.; Tang, B. Z.; He, S. Sci. Rep. 2014, 4, 4279;
(c) Sun, J. B.; Zhang, G. H.; Jia, X. Y.; Xue, P. C.; Jia, J. H.; Lu, R. Acta Chim. Sinica 2016, 74, 165. (孙静波, 张恭贺, 贾小宇, 薛鹏冲, 贾俊辉, 卢然, 化学学报, 2016, 74, 165.);
(d) Wang, C.; Zhang, H.; Tian, L.; Zhu, W.; Lan, Y.; Li, J.; Wang, H.; Zhang, G. X.; Zhang, D. Q.; Yuan, S. L.; Li, G. T. Sci. China Chem. 2016, 59, 89;
(e) Zhao, Z.; Lam, J. W. Y.; Tang, B. Z. Curr. Org. Chem. 2010, 14, 2109;
(f) Gu, X. G.; Yao, J. J.; Zhang, G. X.; Zhang, C.; Yan, Y. L.; Zhao, Y. S.; Zhang, D. Q. Chem.-Asian J. 2013, 8, 2362;
(g) Li, Z. Z.; Huo, Y. P.; Yang, X. H.; Ji, S. M. Chin. J. Org. Chem. 2016, 36, 2317. (李宗植, 霍延平, 阳香华, 籍少敏, 有机化学, 2016, 36, 2317.)
[7] Mei, J.; Hong, Y. N.; Lam, J. W. Y.; Qin, A. J.; Tang, Y. H.; Tang, B. Z. Adv. Mater. 2014, 26, 5429.
[8] He, Z. K.; Zhang, L. Q.; Mei, J.; Zhang, T.; Lam, J. W.Y.; Shuai, Z. G.; Dong, Y. Q.; Tang, B. Z. Chem. Mater. 2015, 27, 6601.
[9] Xu, Y. X.; Wang, K.; Zhang, Y. J.; Xie, Z. Q.; Zou, B.; Ma, Y. G. J. Mater. Chem. C 2016, 4, 1257.
[10] Zhang, Z. Y.; Song, X. X.; Wang, S. P.; Li, F.; Zhang, H. Y.; Ye, K. Q.; Wang, Y. J. Phys. Chem. Lett. 2016, 7, 1697.
[11] Krzeszewski, M.; Thorsted, B.; Brewer, J.; Gryko, D. T. J. Org. Chem. 2014, 79, 3119.
[12] Hisaki, I.; Sakamoto, Y.; Shigemitsu, H.; Tohnai, N.; Miyata, M. Cryst. Growth Des. 2009, 9, 414.
[13] Janiga, A.; Glodkowska-Mrowka, E.; Stoklosa, T.; Gryko, D. T. Asian J. Org. Chem. 2013, 2, 411.
[14] Peng, Z.; Feng, X.; Tong, B.; Chen, D. D.; Shi, J. B.; Zhi, J. G.; Dong, Y. P. Sensor Actuat. B 2016, 232, 264.
[15] Zhu, X. L.; Huang, H.; Liu, R.; Jin, X. D.; Li, Y. H.; Wang, D. F.; Wang, Q.; Zhu, H. J. J. Mater. Chem. C 2015, 3, 3774.
/
| 〈 |
|
〉 |