

Synthesis of InPZnS/ZnS Quantum Dots by Continuous Injection of Phosphorus Precursor
Received date: 2016-10-13
Revised date: 2016-12-27
Online published: 2017-01-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. B21271179, 21607101).
InP quantum dots (QDs) are regarded as the most desirable candidate to replace the role of CdSe QDs in the applications of bio-labeling, LEDs, solar cells, etc, because InP is more environmentally friendly compared to Cd based QDs, and could also offer a tunable emission from blue to near-infrared. Nevertheless, the studies and applications of InP QDs are rather sparse in comparison with CdSe QDs, which are principally caused by significant difficulties in its synthesis. In this report, we developed a novel method for the synthesis of InPZnS/ZnS QDs by using zinc phosphide as phosphorus precursor, and the zinc and sulfur precursors were also added at the start of reaction, which allows the continuous injection of phosphine gas into the reaction, resulting in high quality InPZnS/ZnS quantum dots with emission up to 680 nm. The core synthesis and shell coating were separated by controlling the reaction temperature. During the first 30 minutes, the temperature of reaction solution was kept at 250℃ to grow the InPZnS core QDs. Then, the coating of ZnS shell was happened and kept about 1 hour to guarantee the complete decomposition of 1-dodecanethiol (DDT) after the reaction temperature was increased to 300℃. The biggest advantage of this synthetic method is the tunable emission region from blue to near-infrared. The effects of reaction parameters were systematically investigated. We observed that the molar ratio of In:myristic acid (MA) and that of In:Zn (S) had significant influences on the size of the InP QDs. The structure of InPZnS/ZnS QDs was confirmed by transmission electron microscope (TEM), X-ray powder diffraction (XRD), and energy dispersive X-ray analyzer (EDX). TEM characterization indicated the final core/shell InPZnS/ZnS QDs were good monodispersity with an average size of 7 nm. Furthermore, we investigated the versatility of this method by using other phosphorus precursor. The injection pump leaded to a continuous supply of phosphorus precursor on a timescale and reacted with indium precursor to form InP QDs. The final sample showed an emission at 710 nm. The present method gives access to larger sized InP QDs, making it prosperous for applications in biological labeling.
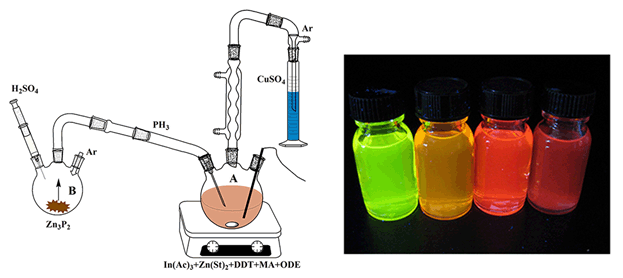
Key words: InPZnS/ZnS; core/shell QDs; quantum yield; optical property; continuous injection
Huang Lu , Li Zhichun , Huang Shouqiang , Peter Reiss , Li Liang . Synthesis of InPZnS/ZnS Quantum Dots by Continuous Injection of Phosphorus Precursor[J]. Acta Chimica Sinica, 2017 , 75(3) : 300 -306 . DOI: 10.6023/A16100543
[1] Bera, D.; Qian, L.; Tseng, T. K.; Holloway, P. H. Materials 2010, 3, 2260.
[2] Bourzac, K. Nature 2013, 493, 283.
[3] Zhang, Y.; Xie, C.; Su, H. P.; Liu, J.; Pickering, S.; Wang, Y. Q.; Yu, W. W.; Wang, J. K.; Wang, Y. D.; Hahm, J.; Dellas, N.; Mohney, S. E.; Xu, J. Nano Lett. 2011, 11, 329.
[4] Michalet, X.; Pinaud, F. F.; Bentolila, L. A.; Tsay, J. M.; Doose, S.; Li, J. J.; Sundaresan, G.; Wu, A. M.; Gambhir, S. S.; Weiss, S. Science 2005, 307, 538.
[5] Li, L.; Daou, J.; Texier, I.; Chi, T. T. K.; Liem, N. Q.; Reiss, P. Chem. Mater. 2009, 21, 2422.
[6] Chen, F. Q.; Gerion, D. Nano Lett. 2004, 4, 1827.
[7] Xie, R. G.; Battaglia, D.; Peng, X. G. J. Am. Chem. Soc. 2007, 129, 15432.
[8] Tamang, S.; Lincheneau, C.; Hermans, Y.; Jeong, S.; Reiss, P. Chem. Mater. 2016, 28, 2491.
[9] Adam, S.; Talapin, D.; Borchert, H.; Lobo, A.; McGinley, C.; De Castro, A.; Hasse, M.; Weller, H.; Möller, T. J. Chem. Phys. 2005, 123, 084706.
[10] Lovingood, D. D.; Strouse, G. F. Nano Lett. 2008, 8, 3394.
[11] Li, Q.; Zhang, T.; Gu, H. W.; Ding, F. Z.; Qu, F.; Peng, X. Y.; Wang, H. Y.; Wu, Z. P. Acta Chim. Sinica 2013, 71, 929. (李谦, 张腾, 古宏伟, 丁发柱, 屈飞, 彭星煜, 王洪艳, 吴战鹏, 化学学报, 2013, 71, 929.)
[12] Li, L.; Reiss, P. J. Am. Chem. Soc. 2008, 130, 11588.
[13] Kim, T. H.; Kim, S. W.; Kang, M. J.; Kim, S. W. J. Phys. Chem. Lett. 2012, 3, 214.
[14] Alt?ntas, Y.; Talpur, M. Y.; Ünlu, M.; Mutlugün, E. J. Phys. Chem. C 2016, 120, 7885.
[15] Li, L.; Protière, M.; Reiss, P. Chem. Mater. 2008, 20, 2621.
[16] Zan, F.; Ren, J. C. J. Mater. Chem. 2012, 22, 1794.
[17] Chen, M. H.; Pan, Z.; Yin, Y. F.; Liu, J.; Liu, M. Y.; Jia, Z. J.; Liang, G. J. Acta Chim. Sinica 2016, 74, 330. (陈美华, 潘峥, 尹月锋, 刘洁, 刘梦媛, 贾紫君, 梁桂杰, 化学学报, 2016, 74, 330.)
[18] Xu, S.; Ziegler, J.; Nann, T. J. Mater. Chem. 2008, 18, 2653.
/
| 〈 |
|
〉 |