

Improving the Reactivity of Zerovalent Iron toward Various Contaminants by Weak Magnetic Field: Performances and Mechanisms
Received date: 2017-01-07
Online published: 2017-02-23
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51478329, 21522704, and U1532120).
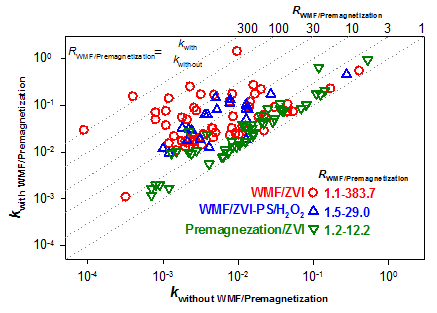
Zero-valent iron (ZVI), a simple but amazingly versatile material, has low intrinsic reactivity toward various contaminants as documented from laboratory studies as well as field demonstrations, which poses potential limitations to its practical application in environmental remediation. Although many methods have been developed to improve the reactivity of ZVI in the literature, high costs, significant work-load, and complex operations may inhibit the application of these methods. We pioneered the research in employing weak magnetic field (WMF) to accelerate the removal of various metal(loid)s, including Se(IV)/Se(VI), As(V)/As(Ⅲ), Sb(V), Cu(Ⅱ)/EDTA-Cu(Ⅱ), and Cr(VI) by pristine ZVI (Pri-ZVI) and/or aged ZVI. The rate constants of metal(loid)s sequestration by Pri-ZVI or aged ZVI were increased by 1.1~383.7 folds due to the application of WMF. Furthermore, WMF could be employed to improve the removal of organic contaminants by ZVI activated H2O2 or persulfate because of the accelerated ZVI corrosion in the presence of WMF. The superimposed WMF had negligible influence on the apparent activation energy of metal(loid)s removal by ZVI, indicating that WMF accelerated metal(loid)s removal by ZVI but did not change the mechanisms. The XAFS, XRD, and XPS analysis confirmed that the application of WMF did not change the mechanisms of metal(loid)s removal but accelerated the transformation (reduction or oxidation) of contaminants. Electrochemical analysis showed that the accelerated ZVI corrosion in the presence of WMF was ascribed to the enhanced mass transfer. We further identified the relative contribution of Lorentz force (FL) and magnetic gradient force (FΔB) in the enhancing effect of WMF. It suggested that FΔB rather than FL was the major driving force for the observed WMF effect on the enhanced reactivity of ZVI. Moreover, we proposed to apply premagnetization to increase the reactivity of ZVI toward As(Ⅲ) sequestration taking advantage of the magnetic memory of ZVI, i.e., the remanence of ZVI. In addition, the premagnetized ZVI (Mag-ZVI) samples from different origins were applied to enhance the removal of various oxidative contaminants[such as azo dyes, As(Ⅲ), Pb(Ⅱ), Cu(Ⅱ), Se(IV), Ag(I) and Cr(VI)] under well-controlled experimental conditions. The rate constants of contaminants removal by premagnetized ZVI samples were 1.2~12.2 folds greater than those by Pri-ZVI samples. As a chemical-and energy-free method, improving the reactivity of ZVI by either WMF superimposition or premagnetization treatment is novel and promising.
Li Jinxiang , Qin Hejie , Zhang Xueying , Guan Xiaohong . Improving the Reactivity of Zerovalent Iron toward Various Contaminants by Weak Magnetic Field: Performances and Mechanisms[J]. Acta Chimica Sinica, 2017 , 75(6) : 544 -551 . DOI: 10.6023/A17010007
[1] Gould, J. P. Water Res. 1982, 16, 871.
[2] Khudenko, B. M. Water Sci. Technol. 1985, 15, 204.
[3] Gillham, R. W.; O'Hannesin, S. F. Ground Water 1994, 32, 958.
[4] Matheson, L. J.; Tratnyek, P. G. Environ. Sci. Technol. 1994, 28, 2045.
[5] EPA, USA, Ground Water Remedies Selected at Superfund Sites, 2002.
[6] Guan, X. H.; Sun, Y. K.; Qin, H. J.; Li, J. X.; Lo, I. M.; He, D.; Dong, H. R. Water Res. 2015, 75, 224.
[7] Wang, C.; Zhang, W. Environ. Sci. Technol. 1997, 94, 9602.
[8] Hung, H. M.; Hoffmann, M. R. Environ. Sci. Technol. 1998, 32, 3011.
[9] Lien, H. L.; Zhang, W. X. J. Environ. Eng. 1999, 125, 1042.
[10] Harendra, S.; Vipulanandan, C. Colloid Surface A 2008, 322, 6.
[11] Liou, Y. H.; Lo, S. L.; Lin, C. J.; Wen, H. K.; Weng, S. C. J. Hazard. Mater. 2005, 126, 189.
[12] Son, H. S.; Im, J. K.; Zoh, K. D. Water Res. 2009, 43, 1457.
[13] Jou, C. J. G.; Hsieh, S. C.; Lee, C. L.; Lin, C.; Huang, H. W. J. Taiwan Inst. Chem. E 2010, 41, 216.
[14] Xu, J.; Hao, Z.; Xie, C.; Lv, X.; Yang, Y.; Xu, X. Desalination 2012, 284, 9.
[15] Huang, Y. H.; Tang, C.; Zeng, H. Chem. Eng. J. 2012, 200, 257.
[16] Scherer, M. M.; Johnson, K. M.; Westall, J. C.; Tratnyek, P. G. Environ. Sci. Technol. 2001, 35, 2804.
[17] Noubactep, C. Environ. Technol. 2008, 29, 909.
[18] Kim, D. H. J. Hazard. Mater. 2011, 192, 928.
[19] Jiang, J. H.; Li, Y. H.; Cai, W. M. J. Hazard. Mater. 2008, 153, 508.
[20] Ambashta, R. D.; Repo, E.; Sillanpää, M. Ind. Eng. Chem. Res. 2011, 50, 11771.
[21] Liang, L.; Sun, W.; Guan, X.; Huang, Y.; Choi, W.; Bao, H.; Li, L.; Jiang, Z. Water Res. 2014, 49, 371.
[22] Liang, L.; Guan, X.; Huang, Y.; Ma, J.; Sun, X.; Qiao, J.; Zhou, G. Sep. Purif. Technol. 2015, 156, Part 3, 1064.
[23] Sun, Y. K.; Guan, X. H.; Wang, J. M.; Meng, X. G.; Xu, C. H.; Zhou, G. M. Environ. Sci. Technol. 2014, 48, 6850.
[24] Guan, X.; Jiang, X.; Qiao, J.; Zhou, G. J. Hazard. Mater. 2015, 300, 688.
[25] Jiang, X.; Qiao, J.; Lo, I. M. C.; Wang, L.; Guan, X.; Lu, Z.; Zhou, G.; Xu, C. J. Hazard. Mater. 2015, 283, 880.
[26] Feng, P.; Guan, X. H.; Sun, Y. K.; Choi, W. Y.; Qin, H. J.; Wang, J. M.; Qiao, J. L.; Li, L. N. J. Environ. Sci.-China 2015, 31, 175.
[27] Li, J.; Bao, H.; Xiong, X.; Sun, Y.; Guan, X. Sep. Purif. Technol. 2015, 151, 276.
[28] Xu, C.; Zhang, B.; Zhu, L.; Lin, S.; Sun, X.; Jiang, Z.; Tratnyek, P. G. Environ. Sci. Technol. 2016, 50, 1483.
[29] Liang, L. P.; Guan, X. H.; Shi, Z.; Li, J. L.; Wu, Y. N.; Tratnyek, P. G. Environ. Sci. Technol. 2014, 48, 6326.
[30] Xu, H.; Sun, Y.; Li, J.; Li, F.; Guan, X. Environ. Sci. Technol. 2016, 50, 8214.
[31] Xiong, X.; Sun, Y.; Sun, B.; Song, W.; Sun, J.; Gao, N.; Qiao, J.; Guan, X. RSC Adv. 2015, 5, 13357.
[32] Xiang, W.; Zhang, B.; Zhou, T.; Wu, X.; Mao, J. Sci. Rep.-UK 2016, 6.
[33] Xiong, X.; Bo, S.; Jing, Z.; Gao, N.; Shen, J.; Li, J.; Guan, X. Water Res. 2014, 62, 53.
[34] Ragsdale, S. R.; Grant, K. M.; White, H. S. J. Am. Chem. Soc. 1998, 120, 13461.
[35] Hinds, G.; Coey, J.; Lyons, M. E. G. Electrochem. Commun. 2001, 3, 215.
[36] Lioubashevski, O.; Katz, E.; Willner, I. J. Phy. Chem. B 2004, 108, 5778.
[37] Waskaas, M.; Kharkats, Y. I. J. Electroanal. Chem. 2001, 502, 51.
[38] Li, J.; Qin, H.; Zhang, W.-X.; Shi, Z.; Zhao, D.; Guan, X. Sep. Purif. Technol. 2016, 176, 40.
[39] Aziz, F.; Pandey, P.; Chandra, M.; Khare, A.; Rana, D. S.; Mavani, K. R. J. Magn. Magn. Mater. 2014, 356, 98.
[40] Ghosh, N.; Mandal, B. K.; Kumar, K. M. J. Magn. Magn. Mater. 2012, 324, 3839.
[41] Li, J. X.; Shi, Z.; Ma, B.; Zhang, P. P.; Jiang, X.; Xiao, Z. J.; Guan, X. H. Environ. Sci. Technol. 2015, 49, 10581.
[42] Li, J.; Qin, H.; Guan, X. Environ. Sci. Technol. 2015, 49, 1440.
[43] Li, X.; Zhou, M.; Pan, Y.; Xu, L. Chem. Eng. J. 2016, 307, 1092.
/
| 〈 |
|
〉 |