

Investigations of Chemical Kinetic Mechanisms for Low-to-medium Temperature Ignition of Ethylene
Received date: 2016-12-06
Online published: 2017-03-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 91441132).
In order to investigate the ignition characteristic of ethylene combustion at low-to-medium temperature, contem-porary detailed kinetic mechanisms for ethylene combustion, including AramcoMech_1.3 mechanism, Creck mechanism, Glarborg's mechanism, San Diego (UCSD) mechanism and Wang's mechanism, were used to simulate ignition delay times of ethylene combustion by Chemkin Pro software reflected shock tube model and closed homogeneous reactor under the as-sumption of constant-volume, homogeneous and adiabatic conditions. Simulated ignition delay times of ethylene combustion using these mechanisms disagree with the experimental data from literatures at low-to-medium temperature.
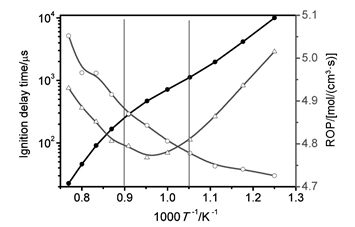
Sensitivity analysis was carried out to identify the controlling steps of C2H4 ignition at 800~1300 K. The sensitivity of ig-nition delay time was calculated by the formula Sensitivity=[τign(2ki)-τign(ki)]/τign(ki)×100%. Here τign(ki) is ignition delay time based on the original combustion mechanism, τign(2ki) is ignition delay time simulated using this mechanism in which the rate constant of reaction i is doubled through multiplying the pre-exponential factor of reaction i by 2. It was demonstrated that C2H3+O2=CH2CHO+O(R1), C2H3+O2=CH2O+HCO(R2) have great sensitivity to the ignition of C2H4 over a wide temperature range, while those reactions involved HO2 (including H2-O2 and C2H4+HO2 system) are important for C2H4 ignition process at low temperature.
By modifying these rate constants of R1 and R2 with more accurate calculated results and adding C2H3+O2=C2H3OO reaction and those reactions involved C2H4+HO2 system, one revised mechanism (UCSD-R2) was obtained. UCSD-R2 mechanism can produce better agreement with recent ethylene ignition delay time experimental data from literatures at low-to-medium temperature compared with UCSD mechanism.
When UCSD-R2 mechanism was adopted to simulate the ignition delay time of ethylene combustion, the first stage ignition delay time at low temperature (800~950 K) and negative temperature coefficient at medium temperature (950~1100 K) were found. They were explained by using sensitivity analysis and rate-of-production analysis. The method of rate-of-production analysis can be employed to calculate the contribution of each reaction to the production and consumption of every species or to calculate total rate-of-production of every species, by Chemkin Pro software closed homogeneous reactor under the assumption of constant-volume, homogeneous and adiabatic conditions. It was demonstrated that C2H4+HO2 system can shorten the ignition delay time obviously, the production and consumption of HO2 radical play a significant role for first stage ignition of C2H4 at low temperature, the consumption of C2H3 radical results in the negative temperature coefficient at medium temperature.
Li Dongyan , Wang Jingbo , Guo Junjiang , Tan Ningxin , Li Xiangyuan . Investigations of Chemical Kinetic Mechanisms for Low-to-medium Temperature Ignition of Ethylene[J]. Acta Chimica Sinica, 2017 , 75(4) : 375 -382 . DOI: 10.6023/A16120656
[1] Shao, J.-X.; Tan, N.-X.; Liu, W.-X.; Li, X.-Y. Acta Phys. Chim. Sin. 2010, 26(2), 270. (邵菊香, 谈宁馨, 刘伟雄, 李象远, 物理化学学报, 2010, 26(2), 270)
[2] Jiang, R.; Liu, G.; Zhang, X. Energ. Fuel. 2013, 27(5), 2563.
[3] Li, J.; Shao, J.-X.; Liu, C.-X.; Rao, H.-B.; Li, Z.-R.; Li, X.-Y. Acta Chim. Sinica 2010, 68(3), 239. (李军, 邵菊香, 刘存喜, 饶含兵, 李泽荣, 李象远, 化学学报, 2010, 68(3), 239.)
[4] Xu, C.; Konnov, A. A. Energy 2012, 43(1), 19.
[5] Suzuki, M.; Moriwaki, T.; Okazaki, S.; Okuda, T.; Tanzawa, T. Astronautica Acta 1973, 18(5), 359.
[6] Cadman, P.; Bambrey, R. J.; Box, S. K.; Thomas, G. O. Combust. Sci. Technol. 2002, 174(11-12), 111.
[7] Liang, J.-H.; Hu, H.-H.; Wang, S.; Zhang, S.-T.; Fan, B.-C.; Cui, J.-P. Chin. J. Theor. Appl. Mech. 2014, 46(1), 155. (梁金虎, 胡弘浩, 王苏, 张胜涛, 范秉诚, 崔季平, 力学学报, 2014, 46(1), 155.)
[8] Kumar, K.; Mittal, G.; Sung, C.; Law, C. Combust. Flame 2008, 153(3), 343.
[9] Metcalfe, W. K.; Burke, S. M.; Ahmed, S. S.; Curran, H. J. Int. J. Chem. Kinet. 2013, 45(10), 638.
[10] Zhou, C.-W.; Li, Y.; O'Connor, E.; Somers, K. P.; Thion, S.; Keesee, C.; Mathieu, O.; Petersen, E. L.; DeVerter, T. A.; Oehlschlaeger, M. A.; Kukkadapu, G.; Sung, C.-J.; Alrefae, M.; Khaled, F.; Farooq, A.; Dirrenberger, P.; Glaude, P.-A.; Bat-tin-Leclerc, F.; Santner, J.; Ju, Y.; Held, T.; Haas, F. M.; Dryer, F. L.; Curran, H. J. Combust. Flame 2016, 167, 353.
[11] Ranzi, E.; Cavallotti, C.; Cuoci, A.; Frassoldati, A.; Pelucchi, M.; Faravelli, T. Combust. Flame 2015, 162(5), 1679.
[12] Lopez, J. G.; Rasmussen, C. L.; Alzueta, M. U.; Gao, Y.; Marshall, P.; Glarborg, P. P. Combust. Inst. 2009, 32(1), 367.
[13] Prince, J. C.; Williams, F. A. Combust. Flame. 2012, 159(7), 2336.
[14] "Chemical-Kinetic Mechanisms for Combustion Applications", San Diego Mechanism web page, Mechanical and Aerospace Engineering (Combustion Research), University of California at San Diego (http://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html).
[15] Guo, J.; Xu, J.; Li, Z.; Tan, N.; Li, X. J. Phys. Chem. A 2015, 119(13), 3161.
[16] Wang, H.; Laskin, A.; Djurisic, Z. M.; Law, C. K.; Davis, S.G.; Zhu, D. L. In Chemical and Physical Processes of Combustion, the 1999 Fall Technical Meeting of the Eastern States Section of the Combustion Institute, Raleigh, NC, October, 1999, pp. 129~132. http://ignis.usc.edu/Mechanisms/C2-C4/c2.html.
[17] Sabia, P.; Joannon, M. d.; Picarelli, A.; Chinnici, A.; Ragucci, R. Fuel. 2012, 91(1), 238.
[18] Merchant, S. S.; Goldsmith, C. F.; Vandeputte, A. G.; Burke, M. P.; Klippenstein, S. J.; Green, W. H. Combust. Flame 2015, 162(10), 3658.
[19] CHEMKIN-PRO 15092, Reaction design, San Diego, 2009.
[20] Kopp, M. M.; Donato, N. S.; Petersen, E. L.; Metcalfe, W. K.; Burke, S. M.; Curran, H. J. J. Propul. Power 2014, 30(3), 790.
[21] Penyazkov, O. G.; Sevrouk, K. L.; Tangirala, V.; Joshi, N. P. Combust. Inst. 2009, 32(2), 2421.
[22] Kopp, M. M.; Petersen, E. L.; Metcalfe, W. K.; Burke, S. M.; Curran, H. J. J. Propul. Power 2014, 30(3), 799.
[23] Goldsmith, C. F.; Harding, L. B.; Georgievskii, Y.; Miller, J. A.; Klippenstein, S. J. J. Phys. Chem. A 2015, 119(28), 7766.
/
| 〈 |
|
〉 |