

Copper Ions Promoted Aerobic Atrazine Degradation by Fe@Fe2O3 Nanowires
Received date: 2017-01-04
Online published: 2017-04-01
Supported by
Project supported by Natural Science Funds for Distinguished Young Scholars (No. 21425728).
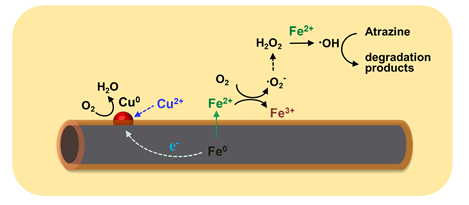
As a persistent chlorinated organic pollutant, Atrazine (2-chloro-4-(ethylamino)-6-isopropylamino-s-triazine) in the environment brings harm to natural environment as well as the human health. Since Atrazine is difficult to be degraded biologically, various strategies have been developed to realize efficient and environmentally-friendly removal of Atrazine. Recently, nanoscaled iron has been extensively applied for the remediation/treatment of wastewater contaminated with various organic and inorganic pollutants and exhibits superior activity than that of bulk iron. But its removal efficiency would decrease along with reaction time. In this study, we report that copper ions could efficiently promote atrazine degradation with Fe@Fe2O3 nanowires via the molecular oxygen activation processes. As indicated by the electron spin resonance analysis (ESR) and X-ray photoelectron spectroscopic analysis (XPS) results, the addition of Cu2+ ions could promote the release of dissolved Fe(Ⅱ) from Fe@Fe2O3. During the degradation process, the concentration of Fe(Ⅱ) in the solution with Cu2+ions is maintained at a much higher level than that without Cu2+ ions. At the same time, Cu2+ions were reduced to low valence states (Cu0), which further promoted the release of Fe2+. The generated Fe2+ would then activate the molecular oxygen via the single-electron or double-electron transfer route. As a result, more reactive oxygen species such as ·OH were generated to degrade atrazine. Under room temperature and aerobic condition, the Atrazine removal rate constant in Fe@Fe2O3/Cu2+ system was 0.694 h-1, which was almost 23 times that in Fe@Fe2O3 system. Moreover, the Fe@Fe2O3/Cu2+catalytic system also remains superior activity in the pH range of 2~5. The intermediates of atrazine degradation were detected and the atrazine degradation in the Fe@Fe2O3/Cu2+ catalytic system was accompanied with alkylic oxidation, dealkylation and dechlorination. This study provides a new way to enhance molecular oxygen activation by core-shell Fe@Fe2O3 nanowires, and also deepens our understanding of the molecular oxygen activation processes by Fe@Fe2O3 for the aerobic pollutant degradation.
Key words: atrazine; degradation; Fe@Fe2O3 nanowires; molecular oxygen activation; copper ions
Jia Falong , Liu Juan , Zhang Lizhi . Copper Ions Promoted Aerobic Atrazine Degradation by Fe@Fe2O3 Nanowires[J]. Acta Chimica Sinica, 2017 , 75(6) : 602 -607 . DOI: 10.6023/A17010004
[1] Hayes, T. B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A. A.; Vonk, A. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 5476.
[2] Dombek, T.; Dolan, E.; Schultz, J.; Klarup, D. Environ. Pollut. 2001, 111, 21.
[3] Rangsivek, R.; Jekel, M. Water Res. 2005, 39, 4153.
[4] Sun, Y.; Li, J.; Huang, T.; Guan, X. Water Res. 2016, 100, 277.
[5] Liu, N.; Zhao, Y. S.; Zhang, L. Y.; Liu, H.; Liu, P. China Environ. Sci. 2006, 26, 116. (刘娜, 赵勇胜, 张兰英, 刘红, 刘鹏, 中国环境科学, 2006, 26, 116.)
[6] Chen, J. L.; Alabed, S. R.; Ryan, J. A.; Li, Z. J. Hazard. Mater. 2001, 83, 243.
[7] Boussahel, R.; Harik, D.; Mammar, M.; Lamara-Mohamed, S. Desalination 2007, 206, 369.
[8] Kim, G.; Jeong, W.; Choe, S. J. Hazard. Mater. 2008, 155, 502.
[9] Liang, L.; Guan, X.; Shi, Z.; Li, J.; Wu, Y.; Tratnyek, P. G. Environ. Sci. Technol. 2014, 48, 6326.
[10] Cheng, R.; Wang, J. L.; Zhang, W. X. Prog. Chem. 2006, 18, 93. (程荣, 王建龙, 张伟贤, 化学进展, 2006, 18, 93.)
[11] Nagpal, V.; Bokare, A. D.; Chikate, R. C.; Rode, C. V.; Paknikar, K. M. J. Hazard. Mater. 2010, 175, 680.
[12] Xu, X. H.; Wei, J. J.; Wang, D. H. China Environ. Sci. 2004, 24, 76. (徐新华, 卫建军, 汪大翚, 中国环境科学, 2004, 24, 76.)
[13] Zhang, W. X. J. Nanopart. Res. 2003, 5, 323.
[14] Li, Y.; Zhang, Y.; Li, J.; Zheng, X. Environ. Pollut. 2011, 159, 3744.
[15] Ai, Z.; Gao, Z.; Zhang, L.; He, W.; Yin, J. J. Environ. Sci. Technol. 2013, 47, 5344.
[16] Huang, Q.; Cao, M.; Ai, Z.; Zhang, L. Appl. Catal. B-Environ. 2015, 162, 319.
[17] Kettle, A. J.; Anderson, R. F.; Hampton, M. B.; Winterbourn, C. C. Biochemistry 2007, 46, 4888.
[18] Wang, L.; Cao, M.; Ai, Z.; Zhang, L. Environ. Sci. Technol. 2014, 48, 3354.
[19] Mills, P.; Sullivan, J. J. Phys. D:Appl. Phys. 1983, 16, 723.
[20] Dean, J. A., Lange's Handbook of Chemistry, 13th ed., McGraw-Hill Book, New York, 1991.
[21] Dong, G.; Ai, Z.; Zhang, L. Water Res. 2014, 66C, 22.
[22] Yang, L.; He, J.; Zhang, Q.; Wang, Y. J. Catal. 2010, 276, 76.
[23] Mudunkotuwa, I. A.; Pettibone, J. M.; Grassian, V. H. Environ. Sci. Technol. 2012, 46, 7001.
[24] Du, C.; Gao, X.; Chen, W. Chin. J. Catal. 2016, 37, 1049. (杜诚, 高小惠, 陈卫, 催化学报, 2016, 37, 1049.)
/
| 〈 |
|
〉 |