

Isolation and Electrochemical Property of Tb@C82 Isomers
Received date: 2017-03-04
Online published: 2017-05-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21271067, 51572071) and the Program for Innovative Research Team in University (No. IRT-1237), Ministry of Education, China.
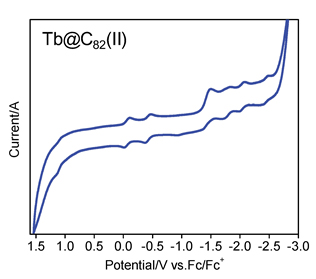
Carbon soot containing terbium endohedral fullerenes was prepared by vaporizing a graphite rod filled with Tb4O7 and graphite powder in an electric arc-discharge chamber. After reflux extraction with 1,2,4-trichlorobenzene and rotary evaporation of solvent, the residue was ultrasonically dissolved in toluene. The obtained solution was filtered with a 0.2 μm PTFE film, and then subjected to a three-stage high-performance liquid chromatographic (HPLC) isolation process to achieve two individual isomers of Tb@C82. The successful isolation of Tb@C82 isomers was confirmed by matrix-assisted laser desorption time-of-flight mass spectrometry and analytical HPLC. According to their UV-Vis-NIR absorption spectra, the carbon cages of the isolated Tb@C82 isomers are estimated to be of C2v and Cs symmetry, respectively, and Tb atom is encapsulated in carbon cage at a valence state of +3. The cyclic voltammetry (CV) of the two Tb@C82 isomers was recorded in a 0.1 mol/L ortho-dichlorobenzene (o-DCB) solution of tetrabutylammonium hexafluorophosphate (TBAPF6) by referring the ferrocene/ferrocenium couple (Fc/Fc+). The working electrode and the counter electrode were platinum wires, and the reference one was a Ag/AgCl electrode. Tb@C82 (I) exhibits two pairs of reversible oxidative and five pairs of reversible reductive peaks, and Tb@C82 (II) shows one reversible oxidative and six reversible reductive ones. It is found that the symmetry of C82 cage has little effect on the reduction potentials but great effect on the oxidation potentials of Tb@C82 isomers. Moreover, the first reduction potential of both Tb@C82 isomers is relatively high, indicating that they are good electron accepting materials. Additionally, the corresponding redox potentials of Tb@C82 (II) are relatively higher than those of Tb@C82 (I), therefore it is concluded that Tb@C82 (II) is of better electron accepting ability than Tb@C82 (I). In comparison with those reported previously, three pairs of reversible reductive and one pair of reversible oxidative peaks were initially detected for Tb@C82 (I), offering more information on the electrochemistry of metallofullerene.
Key words: metallofullerene; terbium; isomer; electrochemistry; redox potential
Dong Wei , Nie Mengsi , Lian Yongfu . Isolation and Electrochemical Property of Tb@C82 Isomers[J]. Acta Chimica Sinica, 2017 , 75(5) : 453 -456 . DOI: 10.6023/A17030090
[1] (a) Jiang, L.; Wang, T.-S.; Shu, C.-Y.; Wang, C.-R. Sci. China-Chem. 2011, 41, 629 (in Chinese). (蒋礼, 王太山, 舒春英, 王春儒, 中国科学:化学, 2011, 41, 629.)
(b) Wang, Z.; Nakanishi, Y.; Noda, S.; Niwa, H.; Zhang, J.; Kitura, R.; Shinohara, H. Angew. Chem., Int. Ed. 2013, 125, 11986.
(c) Nakanishi, Y.; Omachi, H.; Matsuura, S.; Miyata, Y.; Kitaura, R.; Itami, K.; Shinohara, H. Angew. Chem., Int. Ed. 2014, 53, 3102.
[2] (a) Xie, Q.; Arias, R.; Echegoyen, L. J. Am. Chem. Soc. 1993, 775, 9818.
(b) Alexey, A.; Shangfeng, Y.; Lothar, D. Chem. Rev. 2013, 113, 5989.
[3] Lu, X.; Slanina, Z.; Akasaka, T.; Tsuchiya, T.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2010, 132, 5896.
[4] Suzuki, T.; Kikuchi, K.; Oguri, F.; Nakao, Y.; Suzuki, S.; Achiba, Y.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Tetrahedron 1996, 52, 4973.
[5] Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Chem. Soc. Rev. 2012, 41, 7723.
[6] (a) Akasaka, T.; Kono, T.; Matsunaga, Y.; Wakahara, T.; Nakahodo, T.; Ishitsuka, M. O.; Maeda, Y.; Tsuchiya, T.; Kato, T.; Liu, M. T. H.; Mizorogi, N.; Slanina, Z.; Nagase, S. J. Phys. Chem. A 2008, 112, 1294.
(b) Suzuki, T.; Maruyama, Y.; Kato, T.; Kikuchi, K.; Achiba, Y. J. Am. Chem. Soc. 1993, 115, 11006.
[7] (a) Akasaka, T.; Okubo, S.; Kondo, M.; Maeda, Y.; Wakahara, T.; Kato, T.; Suzuki, T.; Yamamoto, K.; Kobayashi, K.; Nagase, S. Chem. Phys. Lett. 2000, 319, 153.
(b) Kikuchi, K.; Nakao, Y.; Suzuki, S.; Achiba, Y.; Suzuki, T.; Maruyama, Y. J. Am. Chem. Soc. 1994, 116, 9367.
[8] Xu, J. X.; Li, M. X.; Shi, Z. J.; Gu, Z. N. Chem. Eur. J. 2005, 12, 562.
[9] (a) Bao, L.-P.; Pan, C.-W.; Slanina, Z.; Uhlik, F.; Akasaka, T.; Lu, X. Angew. Chem., Int. Ed. 2016, 55, 9234.
(b) Maeda, Y.; Matsunaga, Y.; Wakahara, T.; Takahashi, S.; Tsuchiya, T.; Ishitsuka, M.; Hasegana, T.; Akasaka, T.; Liu, M.; Kokura, K.; Horn, E.; Yoza, K.; Kato, T.; Okubo, S.; Kobayashi, K.; Nagase, S.; Yamamoto, K. J. Am. Chem. Soc. 2004, 126, 6858.
[10] Fan, L.-Z.; Wu, Y.-Q.; Yang, S.-F.; Yang, S.-H. Acta Chim. Sinica 2004, 62, 2213 (in Chinese). (范楼珍, 吴月芹, 杨尚峰, 杨世和, 化学学报, 2004, 62, 2213.)
[11] Wang, W.; Ding, J.; Yang, S.-F.; Li, X.-Y. Electrochemical Properties of 4f-Block Metallofullerenes. In Fullerenes. Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials, Vol. 4, Eds.: Kadish, K. M.; Ruoff, R. S., Electrochemical Society, Pennington, 1997, p. 417.
[12] (a) Liu, F.-P.; Wang, S.; Guan, J.; Wei, T.; Zeng, M.; Yang, S.-F. Inorg. Chem. 2014, 53, 5201.
(b) Liu, F.-P.; Gao, C.-L.; Deng, Q.-M.; Zhu, X.-J.; Kostanyan, A.; Westerstrom, R.; Wang, S.; Tan Y.-Z.; Tao, J.; Xie, S.-Y.; Popov, A.; Greber, T.; Yang, S.-F. J. Am. Chem. Soc. 2016, 138, 14764.
[13] Hino, S.; Umishita, K.; Iwasaki, K.; Aoki, M.; Kobayashi, K.; Nagase, S.; Dennus, T.-J.-S.; Nakane, T.; Shinohara, H. Chem. Phys. Lett. 2001, 337, 65.
/
| 〈 |
|
〉 |