

Lignin C-C Bond's Cleavage by Vanadium Catalyzed with High Selectivity in Acid Environment
Received date: 2017-05-08
Online published: 2017-06-15
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21325208,21272050,21402181,21572212),the Index Program Directive Foundation of Hefei Centre for Physical Science and Technology (No.2014FXCX006),the Science Foundation of the Chinese Academy of Sciences (Nos.KFJ-EW-STS-051,XDB20000000,YZ201563),the Specialized Research Fund for the Doctoral Program of Higher Education (No.20123402130008),the Fundamental Research Funds for the Central Universities (Nos.WK2060190025,WK2060190040),the Key Technologies R&D Program of Anhui Province (No.1604a0702027) and the Program for Changjiang Scholars and Innovative Research Team in University of the Ministry of Education of China.
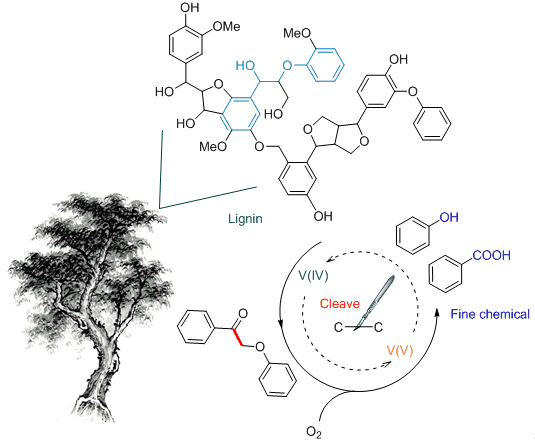
Lignin is a potential resources of aromatic compound that can be obtained from renewable biomass. There are many ongoing research efforts to utilize lignin as a sustainable alternative to petroleum derived aromatic compounds. Because of the complex three-dimensional structure, the depolymerization of lignin into monomer molecule became a core challenge for the utilization of lignin. The β-O-4 structure is the most abundant linkage in lignin. Owing to its abundance, the β-O-4 structure has been representatively studied in many aspects of scientific research on lignin degradation. Among the different reported strategies for the cleavage of β-O-4 ether bonds, C-C bond cleavage is one of the most important approaches to depolymerizing lignin. In this study, we accomplished the oxidative C-C bond cleavage of the β-O-4 structure by the catalysis of NH4VO3 using the pre-oxidized 2-phenoxy-1-phenylethanone (1a) as a model compound of lignin. In the DMSO-HOAc solvent system, benzoic acid and phenol were produced in a moderate condition, the yeild of benzoic acid and phenol were 82.1% and 88.1%, respectively. The reaction process was investigated via 1H NMR and X-ray photoelectron spectra (XPS) characterizations and the possible reaction pathway was further proposed. As the results shown, two possible reaction routes existed in this catalytic system. Pathway one:the 2-hydroxyacetophenone and phenol formed after the C-O bond cleavage of 1a in the acidic system, then, the intermediate 2-hydroxyacetophenone was converted to benzoic via the cleavage of C-C bond. Pathway two:benzoic acid and phenol yielded by the C-C bond of 1a cleaved directly over the catalyst. In addition, the catalyst characterization results confirmed that the oxovanadium(V) directly catalyzed the depolymerization of the β-O-4 structure and generated oxovanadium(IV), then oxovanadium(IV) was oxidized by O2 and finish the catalytic cycle. All reactions were carried out by the following general procedure. This reaction was carried out in glass tube and heated by oil bath. 0.5 mmol of 1a was added into 2 mL of DMSO-HOAc (V:V=3:1) with 30 mol% NH4VO3 (17.5 mg) under an oxygen atmosphere (101 kPa, in balloon). The reactor was heated to 100℃ with a powerful stirring. After 8 h, the reaction was cooled to room temperature, then 5 mL of ethyl acetate was added into the mixtures. Ash black precipitate was removed by filtration and the liquid mixture was detected by GC.
Liu Xinxin , Yan Long , Fu Yao . Lignin C-C Bond's Cleavage by Vanadium Catalyzed with High Selectivity in Acid Environment[J]. Acta Chimica Sinica, 2017 , 75(8) : 788 -793 . DOI: 10.6023/A17050199
[1] Corma, A.; Iborra, S.; Velty, A. Chem. Rev. 2007, 107, 2411.
[2] (a) Delidovich, I.; Hausoul, P. J. C.; Deng, L.; Pfuzenreuter, R.; Rose, M.; Palkovits, R. Chem. Rev. 2016, 116, 1540.
(b) Yang, Z.; Fu, Y.; Guo, Q. X. Chin. J. Org. Chem. 2015, 35, 273(in Chinese). (杨珍, 傅尧, 郭庆祥, 有机化学, 2016, 35, 273.)
[3] (a) Ragauskas, A. J.; Williams, C. K.; Davison, B. H.; Britovsek, G.; Cairney, J.; Eckert, C. A.; Hallett, J. P.; Leak, D. J.; Liotta, C. L.; Mielenz, J. R.; Murphy, R.; Templer, R.; Tschaplinski. T. Science 2006, 311, 484.
(b) Regalbuto, J. R. Science 2009, 325, 822.
(c) Holm, M. S.; Saravanamurugan, S.; Taarning, E. Science 2010, 328, 602.
(d) Luo, C.; Wang, S.; Liu, H. Angew. Chem., Int. Ed. 2007, 46, 7636.
(e) Wang, H. J.; Zhao, Y.; Wang, C.; Fu, Y.; Guo, Q. X. Acta Chim. Sinica 2009, 67, 893(in Chinese). (王华静, 赵岩, 王晨, 傅尧, 郭庆祥, 化学学报, 2009, 67, 893.)
[4] (a) Xu, C. P.; Arancon, R. A. D.; Labidi, J.; Luque, R. Chem. Soc. Rev. 2014, 43, 7485.
(b) Upton, B. M.; Kasko, A. M. Chem. Rev. 2016, 116, 2275.
(c) Li, C. Z.; Zhao, X. C.; Wang, A. Q.; Huber, G. W.; Zhang, T. Chem. Rev. 2015, 115, 11559.
(d) Deng, W. P.; Zhang, H. X.; Xue, L. Q.; Zhang, Q. H.; Wang, Y. Chin. J. Catal. 2015, 36, 1440.
[5] (a) Zaheer, M.; Kempe, R. ACS Catal. 2015, 5, 1675.
(b) Zeng, W. P.; Li, X. H.; Du, J.; Li, J. M.; Zhang, P.; Hu, C. W.; Meng, X. G. Acta Chim. Sinica 2010, 68, 27(in Chinese). (曾伟鹏, 李小红, 杜娟, 李建梅, 张平, 胡常伟, 孟祥光, 化学学报, 2010, 68, 27.)
[6] (a) Sergeev, A. G.; Hartwig, J. F. Science 2011, 332, 439.
(b) Sergeev, A. G.; Webb, J. D.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 20226.
[7] (a) Zhou, C. H.; Xia, X.; Lin, C. X.; Tong, D. S.; Berltramini, J. Chem. Soc. Rev. 2011, 40, 5588.
(b) Wang, W. L.; Wang, J. Y.; Dong, X. Z.; Chen, P. Chem. Bull. 2016, 79, 731(in Chinese). (王唯黎, 王景芸, 董晓哲, 陈平, 化学通报, 2016, 79, 731.)
[8] Shiramizu, M.; Toste, F. D. Angew. Chem., Int. Ed. 2012, 51, 8082.
[9] Zakzeski, J.; Bruijnincx, P. C. A.; Jongerius, A. L.; Weckhuysen, B. M. Chem. Rev. 2010, 110, 3552.
[10] Alonso, D. M.; Wettstein, S.; G. Dumesic, J. A. Chem. Soc. Rev. 2012, 41, 8075.
[11] (a) Zhang, H. F.; Yang, J. Y.; Wu, J. X.; Mao, H. F.; Sun, X. L. Chin. J. Org. Chem. 2016, 36, 1266(in Chinese). (张海峰, 杨军艳, 吴建新, 毛海舫, 孙小玲, 有机化学, 2016, 36, 1266.)
(b) Ouyang, X. P.; Yang, Y.; Zhu, G. D.; Qiu, X. Q. Chin. Chem. Lett. 2015, 26, 980.
[12] (a) He, J.; Zhao, C.; Lercher, J. A. J. Am. Chem. Soc. 2012, 134, 20768.
(b) Song, Q.; Wang, F.; Cai, J. Y.; Wang, Y. H.; Zhang, J. J.; Yu, W. Q.; Xu, J. Energy Environ. Sci. 2013, 6, 994;
(c) Wang, X.; Rinaldi, R. ChemSusChem 2012, 5, 1455.
(d) Sturgeon, M. R.; O'Brien, M. R.; Ciesielski, P. N.; Katahira, R.; Kruger, J. S.; Chmely, S. C.; Hamlin, J.; Lawrence, K.; Hunsinger, G. B.; Foust, T. D.; Baldwin, R. M.; Biddy, M. J.; Beckham, G. T. Green Chem. 2014, 16, 824.
(e) Song, Q.; Cai, J. Y.; Zhang, J. J.; Yu, W. Q.; Wang, F.; Xu, J. Chin. J. Catal. 2013, 34, 651.
[13] Ren, Y. L.; Yan, M. J.; Wang, J. J.; Zhang, Z. C.; Yao, K. S. Angew. Chem., Int. Ed. 2013, 52, 12674.
[14] Harm, R. G.; Markovits, I.-I. E.; Drees, M.; Mult, H. C.; Herrmann, W. A.; Cokoja, M.; Kuhn, F.-E. ChemSusChem 2014, 7, 429.
[15] Nichols, J. M.; Bishop, L. M.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2010, 132, 12554.
[16] (a) Zhou, X. Y.; Mitra, J.; Rauchfuss, T. B. ChemSusChem 2014, 7, 1623.
(b) Yan, L.; Pang, H.; Huang, Y. B.; Fu, Y. Acta Chim. Sinica 2014, 72, 1005(in Chinese). (严龙, 庞欢, 黄耀兵, 傅尧, 化学学报, 2014, 72, 1005.)
[17] (a) Guo, H. W.; Zhang, B.; Li, C. Z.; Peng, C.; Dai, T.; Xie, H. B.; Wang, A. Q.; Zhang, T. ChemSusChem 2016, 9, 3220.
(b) Heather, J.; Parker, H. J.; Chuck, C. J.; Woodman, T.; Jones, M. D. Catal. Today 2016, 269, 40.
(c) Xiao, Y. W.; Xiu, Y. L. Chin. J. Chem. 2011, 22, 733.
[18] (a) Hanson, S. K.; Baker, R. T.; Gordon, J. C.; Scott, B. L.; Thorn, D. L. Inorg. Chem. 2010, 49, 5611.
(b) Hanson, S. K.; Wu, R.; Silks, L. A. P. Angew. Chem., Int. Ed. 2012, 51, 3410.
(c) Zhang, G.; Scott, B. L.; Wu, R. L.; Silks, L. A. P.; Hanson, S. K. Inorg. Chem. 2012, 51, 7354.
(d) Sedai, B.; Urrutia, C. D.; Baker, R. T.; Wu, R. L.; Silks, L. A. P.; Hanson, S. K. ACS. Catal. 2013, 3, 3111.
(e) Diaz-Urrutia, C.; Sedai, B.; Leckett, K. C.; Baker, R. T.; Hanson, S. ACS Sustanable Chem. Eng. 2016, 4, 6244.
[19] (a) Ma, Y. Y.; Du, Z. T.; Liu, J. X.; Xia, F.; Xu, J. Green Chem. 2015, 17, 4968.
(b) Ma, Y. Y.; Du, Z. T.; Xia, F.; Ma, J. P.; Gao, J.; Xu, J. RSC Adv. 2016, 6, 110229.
[20] (a) Gazi, S.; Ng, W. K. H.; Ganguly, R.; Moeljadi, A. M. P.; Soo, H. S. Chem. Sci. 2015, 6, 7130.
(b) Mottweiler, J.; Puche, M.; Rauber, C.; Schmidt, T.; Concepcion, P.; Corma, A.; Bolm, C. ChemSusChem 2015, 8, 1206.
[21] (a) Mottweiler, J.; Rinesch, T.; Besson, C.; Buendia, J.; Bolm, C. Green Chem. 2015, 17, 5001.
(b) Stein, T V.; Hartog, T. D.; Buendia, J.; Stoychev, S.; Mottweiler, J.; Bolm, C.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2015, 54, 5859.
(c) Deng, W. P.; Zhang, H. X.; Wu, X. J.; Li, R. S.; Zhang, Q. H.; Wang, Y. Green Chem. 2015, 17, 5009.
(d) Mitchell, L. J.; Moody, C. J. J. Org. Chem. 2014, 79, 11091.
[22] (a) Rahimi, A.; Ulbrich, A.; Coon, J. J.; Stahl, S. S. Nature 2015, 515, 249.
(b) Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 6415.
[23] (a) Patil, N. D.; Yan, N. Catal. Commun. 2016, 84, 155.
(b) Yao, S. G.; Meier, M. S.; Pace Ⅲ, R. B.; Crocker, M. RSC Adv. 2016, 6, 104742.
(c) Mobley, J. K.; Yao, S. G.; Crocker, M.; Meier, M. RSC Adv. 2015, 5, 105136.
(d) Nguyen, J. D.; Matsuura, B. S.; Stepemson, C. R. J. J. Am. Chem. Soc. 2014, 136, 1218.
(e) Luo, J.; Zhang, J. J. Org. Chem. 2016, 81, 9131.
(f) Karakas, D. M.; Bosque, I.; Stephenson, C. R. J. Org. Lett. 2016, 18, 5166.
[24] (a) Wang, M.; Lu, J. M.; Zhang, X. C.; Li, L. H.; Li, Hong. J.; Luo, N. C.; Wang, F. ACS Catal. 2016, 6, 6086.
(b) Wang, M.; Li, L. H.; Lu, J. M.; Li, H. J.; Zhang, X. C.; Liu, H. F.; Luo, N. C.; Wang, F. Green Chem. 2017, 19, 702.
(c) Liu, H. F.; Wang, M.; Li, H. J.; Luo, N. C.; Xu, S. T.; Wang, F. J. Catal. 2017, 346, 170.
[25] (a) Yang, Y. Y.; Fan, H. L.; Song, J. L.; Meng, Q. L.; Zhou, H. C.; Wu, L. Q.; Yang, G. Y.; Han, B. X. Chem. Coummn. 2015, 51, 4028.
(b) Paitil, N. D.; Yan, N. Tetrahedron Lett. 2016, 57, 3024.
[26] (a) Luo, N. C.; Wang, M.; Li, H.; Zhang, J.; Liu, H.; Wang, F. ACS Catal. 2016, 6, 7716.
(b) Zhang, J.; Liu, Y.; Chiba, S.; Loh, T.-P. Chem. Commun. 2013, 49, 11439.
[27] Dakkach, M.; Atlamsani, A.; Sebti, S. C. R. Chim. 2012, 15, 482.
[28] (a) Wang, W. H.; Niu, M.; Hou, Y. C.; Wu, W. Z.; Liu, Z. Y.; Liu, Q. Y.; Ren, S. H.; Marsh, K. N. Green Chem. 2014, 16, 2614.
(b) Niu, M.; Hou, Y.-C.; Ren, S. H.; Wang, W. H.; Zheng, Q. T.; Wu, W. Z. Green Chem. 2015, 17, 335.
(c) Niu, M.; Hou, Y. C.; Ren, S. H.; Wu, W. Z.; Marsh, K. N. Green Chem. 2015, 17, 453.
[29] (a) Custodis, V. B. F.; Karakoulia, S. A.; Triantafyllidis, K. S.; van Bokhoven, J. A. ChemSusChem 2016, 9, 1134.
(b) Shu, R. Y.; Xu, Y.; Zhang, Q.; Ma, L. L.; Wang, T. J. CIESC J. 2016, 67, 4523(in Chinese). (舒日洋, 徐莹, 张琦, 马隆龙, 王铁军, 化工学报, 2016, 67, 4523.)
[30] (a) Shuai, L.; Amiri, M. T.; Questell-Santiago, Y. M.; Heroguel, F.; Li, Y. D.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J. Science 2016, 354, 329.
(b) Wang, L.; Meng, Y. Z.; Wang, S. J.; Shang, X. Y.; Li, L.; Hay, A. S. Macromolecules 2004, 37, 3151.
[31] Huang, X. Q.; Li, X. Y.; Zou, M. C.; Song, S.; Tang, C. H.; Yuan, Y. Z.; Jiao, N. J. Am. Chem. Soc. 2014, 136, 14858.
[32] Jiang, Y. Y.; Yan, L.; Zhang, Q.; Fu, Y. ACS Catal. 2016, 6, 4399.
/
| 〈 |
|
〉 |