

Preparation of Crack-free Inverse-opal Films by Template/Matrix Co-assembly
Received date: 2017-05-31
Online published: 2017-09-04
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21401154, U1405226), the 111 Project (No. B16029), the Natural Science Foundation of Guangdong Province (2014A030310005) and the Fundamental Research Funds for the Central Universities of China (No. 20720170011).
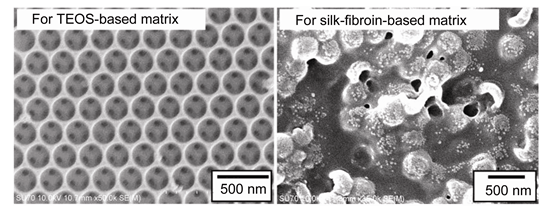
Recently, there has been a significant interest in utilizing well-ordered, porous inverse-opal films for applications in optical, electronic and (bio)chemical fields. However, uncontrolled defects are always formed during their preparation process, which limit their practical applications. In this work, we examine the feasibility of using template/matrix co-assembly strategies to fabricate crack-free inverse opal thin films. Polystyrene spheres (PS) are chosen as a colloidal template, and two matrix precursors[tetraethoxysilane (TEOS) precursor and regenerated silk fibroin solution] are used for the current study. Our scanning electron microscope (SEM) and optical spectrum results show that, for the TEOS-based system, the resulting silica gel due to the sol-gel transition of TEOS can effectively fill the gap between particles, but cannot affect the self-assembly of PS colloidal particles. After selective removal of the PS template, centimeter-scale crack-free and well-ordered inverse opal films can be obtained. In addition, for a constant concentration of TEOS, the film thickness and order degree of structure can be simply tuned by adjusting the concentrations of colloidal spheres. In comparison with indirect approach through template self-assembly and liquid infiltration, such a co-assembly approach can effectively minimize the associated cracking and avoid the need for matrix infiltration into the preassembled colloidal spheres. On the other hand, macro-molecule silk fibroin has a relatively strong interaction with PS colloidal particles, which is demonstrated by SEM and confocal images. Due to their interaction, silk fibroin molecules are preferably adsorbed on the surface of PS spheres, which can restrain the self-assembly of colloidal particles. As a result, it cannot form well-ordered silk film based on such co-assembly strategy. That is to say, the co-assembly approach is not suitable for systems that matrices have strong interactions with templates. These findings pave the way to use the template/matrix co-assembly strategy for rationally designing and developing crack-free inverse opal films and to apply such well-ordered and porous materials in a variety of fields.
Key words: co-assembly; inverse-opal structure; crack-free; colloidal spheres
Luo Wenhao , Zhu Shuihong , Lin Youhui , Liu Xiang Yang . Preparation of Crack-free Inverse-opal Films by Template/Matrix Co-assembly[J]. Acta Chimica Sinica, 2017 , 75(10) : 1010 -1016 . DOI: 10.6023/A17050236
[1] Whitesides, G. M.; Grzybowski, B. Science 2002, 295, 2418.
[2] Zhao, X.; Su, F.; Yan, Q.; Guo, W.; Bao, X. Y.; Lv, L.; Zhou, Z. J. Mater. Chem. 2006, 16, 637.
[3] Holland, B. T.; Blanford, C. F.; Stein, A. Science 1998, 281, 538.
[4] Arsenault, A. C.; Clark, T. J.; von Freymann, G.; Cademartiri, L.; Sapienza, R.; Bertolotti, J.; Vekris, E.; Wong, S.; Kitaev, V.; Manners, I. Nat. Mater. 2006, 5, 179.
[5] Rinne, S. A.; García-Santamaría, F.; Braun, P. V. Nat. Photonics 2008, 2, 52.
[6] Choi, S. W.; Xie, J.; Xia, Y. Adv. Mater. 2009, 21, 2997.
[7] Lee, K.; Asher, S. A. J. Am. Chem. Soc. 2000, 122, 9534.
[8] Li, Y.; Qi, L.-M. Acta Chim. Sinica 2015, 73, 869. (李扬; 齐利民, 化学学报, 2015, 73, 869.)
[9] Hatton, B.; Mishchenko, L.; Davis, S.; Sandhage, K. H.; Aizenberg, J. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 10354.
[10] Lytle, J. C.; Stein, A. Annual Review of Nano Research, Vol. 1, Eds.:Cao, G. Z.; Brinker, C. J., World Scientific Publishing Co., Singa-pore, 2006, 1, pp. 1~14.
[11] Velev, O. D.; Lenhoff, A. M. Curr. Opin. Colloid. Interface Sci. 2000, 5, 56.
[12] Jiang, F.-G.; Yao, J.-R.; Chen, X.; Shao, Z.-Z. Acta Chim. Sinica 2009, 67, 1675. (蒋伏广, 姚晋荣, 陈新, 邵正中, 化学学报, 2009, 67, 1675.)
[13] Tu, H.; Yu, R.; Lin, Z.; Zhang, L.; Lin, N.; Yu, W. D.; Liu, X. Y. Adv. Funct. Mater. 2016, 26, 9032.
[14] Ke, G.-Z.; Xie, H.-F.; Ruan, R.-P.; Yu, W.-D. Energy Convers. Manage. 2010, 51, 2294.
[15] Liu, R.; Wan, L.; Liu, S.; Pan, L.; Wu, D.; Zhao, D. Adv. Funct. Mater. 2015, 25, 526.
[16] Chen, Z.; Zhang, H.; Lin, Z.; Lin, Y.; van Esch, J. H.; Liu, X. Y. Adv. Funct. Mater. 2016, 26, 8978.
[17] Nagarkar, S.; Nicolai, T.; Chassenieux, C.; Lele, A. Phys. Chem. Chem. Phys. 2010, 12, 3834.
[18] Cao, H.; Chen, X.; Shao, Z.-Z. Acta Chim. Sinica 2008, 66, 2059. (曹惠, 陈新, 邵正中, 化学学报, 2008, 66, 2059.)
[19] Schroden, R. C.; Al-Daous, M.; Blanford, C. F.; Stein, A. Chem. Mater. 2002, 14, 3305.
[20] Diao, Y. Y.; Liu, X. Y.; Toh, G. W.; Shi, L.; Zi, J. Adv. Funct. Mater. 2013, 23, 5373.
[21] Wong, S.; Kitaev, V.; Ozin, G. A. J. Am. Chem. Soc. 2003, 125, 15589.
[22] Zhou, Z.; Zhao, X. Langmuir 2005, 21, 4717.
[23] Zhang, T. H.; Kuipers, B. W.; Groenewold, J.; Kegel, W. K. Soft Matter. 2015, 11, 297.
[24] Zhang, T. H.; Liu, X. Y. Chem. Soc. Rev. 2014, 43, 2324.
[25] Chabanov, A. A.; Jun, Y.; Norris, D. J. Appl. Phys. Lett. 2004, 84, 3573.
[26] Huang, Y.; Zhou, J.; Su, B.; Shi, L.; Jiang, L. J. Am. Chem. Soc. 2012, 134, 17053.
[27] Busch, K.; John, S. Phys. Rev. E 1998, 58, 3896.
/
| 〈 |
|
〉 |