

Highly Sensitive Detection of Mercury Ion Based on Plasmon Coupling
Received date: 2017-06-30
Online published: 2017-09-04
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21327902, 21535003).
Mercury is very harmful to the environment and human health even at low concentration. Methods for sensitive detection of mercury ion (Hg2+) have increasingly been developed over the past decade owing to the rapid development in nanotechnology. However, the limits of detection (LODs) of these methods are mostly not satisfactory enough to meet the demand of monitoring trace amounts of mercury ion. DNA thymine (T bases) can react with the mercury ion to form T-Hg2+-T structure, and this interaction has been proved to be much more stable than the interaction between thymine and its complementary adenine (A bases). Based on this principle, a series of ultra-sensitive DNA-based colorimetric biosensors, mostly using Au nanoparticles (AuNPs) as DNA carriers, have been designed for detection of mercury ion. In this study, we report a new strategy for highly sensitive Hg2+ detection based on Hg2+-induced AuNPs assembly. AuNPs of different sizes (s-AuNPs of 18 nm and c-AuNPs of 52 nm) were modified with oligonucleotides containing a sequence of continuous T bases. In the presence of Hg2+, s-AuNPs would be bound to c-AuNPs in the solution owing to oligonucleotide hybridization, forming a core-satellites nanostrucure. This process was accompanied by a color change of the scattering light from green to orange as observed under dark-field microscopy and a corresponding distinct scattering peak shift. The scattering spectra of the AuNPs were obtained using a spectroscopic system which was established autonomously. The scattering peak shift of color-changed spots corresponded with Hg2+ concentration. It was increased linearly with logarithm of Hg2+ concentration over a wide range from 1 pmol/L to 1 nmol/L, with the correlation coefficient of 0.983 (R2=0.983), and the detection limit of Hg2+ was estimated to be 1 pmol/L. Other metal ions, such as Ag+, K+, Ca2+, Mg2+, Zn2+, Cd2+, Fe2+, Pb2+, Ni2+, Mn2+, Al3+, induced negligible scattering peak shifts for AuNPs under the same conditions, which showed that this strategy exhibited excellent selectivity towards Hg2+. Moreover, satisfactory results were obtained when the proposed approach was applied to detect Hg2+ in real samples with recoveries of 98.7%~103.1% and 105.6%~108.2% for river water and tap water, respectively.
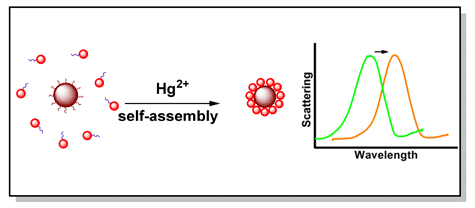
Key words: mercury ion; nanoassembly; T-Hg2+-T; plasmon coupling
Qian Guangsheng , Zhao Wei , Xu Jingjuan , Chen Hongyuan . Highly Sensitive Detection of Mercury Ion Based on Plasmon Coupling[J]. Acta Chimica Sinica, 2017 , 75(11) : 1097 -1102 . DOI: 10.6023/A17060290
[1] Li, Y.; Jing, C.; Zhang, L.; Long, Y. T. Chem. Soc. Rev. 2012, 41, 632.
[2] Anker, J. N.; Hall, W. P.; Lyandres, O.; Shah, N. C.; Zhao, J.; Van Duyne, R. P. Nature Mater. 2008, 7, 442.
[3] Zhang, L.; Sheng, J. J.; Fan, Q. L.; Wang, L. H.; Huang, W. Chin. Sci. Bull. 2014, 59, 169(in Chinese). (张磊, 沈晶晶, 范曲立, 汪联辉, 黄维, 科学通报, 2014, 59, 169.)
[4] Wang, Y. J.; Zu, X. H.; Yi, G. B.; Luo, H. S.; Huang, H. L.; Song, X. L. Chin. J. Chem. 2016, 34, 1321.
[5] Li, Y.; Lin, Z.; Li, R. Z.; Liu, X. Acta Chim. Sinica 2012, 70, 1304(in Chinese). (李迎, 林钊, 李蓉卓, 刘霞, 化学学报, 2012, 70, 1304.)
[6] Kelly, K. L.; Coronado, E.; Zhao, L. L.; Schatz, G. C. J. Phys. Chem. B 2003, 107, 668.
[7] Hartland, G. V. Chem. Rev. 2011, 111, 3858.
[8] Sonnichsen, C.; Reinhard, B. M.; Liphardt, J.; Alivistos A. P. Nat. Biotechnol. 2005, 23, 741.
[9] Sheikholeslami, S.; Jun, Y.; Jain, P. K.; Alivistos, A. P. Nano Lett. 2010, 10, 2655.
[10] Jun, Y.-W.; Sheikholeslami, S.; Hostetter, D. R.; Tajon, C.; Craik, C. S.; Alivistos A. P. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 17735.
[11] Reinhard, B. M.; Sheikholeslami, S.; Mastroianni, A.; Alivistos, A. P.; Liphardt, J. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 2667.
[12] Zhen, S. J.; Wan, X. Y.; Zheng, L. L.; Li, C. M.; Huang, C. Z. Sci. Bull. 2016, 61, 639.
[13] Li, X.-L.; Zhang, Z.-L.; Zhao, W.; Xia, X.-H; Xu, J.-J.; Chen, H.-Y. Chem. Sci. 2016, 7, 3256.
[14] Lermusiaux, L.; Maillard, V.; Bidault, S. ACS Nano 2015, 9, 978.
[15] Yoon, J. H.; Lim, J.; Yoon, S. ACS Nano 2012, 6, 7199.
[16] Qian, G. S.; Kang, B.; Zhang, Z. L.; Li, X. L.; Zhao, W.; Xu, J. J.; Chen, H. Y. Chem. Commun. 2016, 52, 11052.
[17] Clarkson, T. W.; Magos, L.; Myers, G. J. N. Engl. J. Med. 2003, 349, 1731.
[18] Ma, X.; Song, F.; Wang, L.; Cheng, Y.; Zhu, C. J. Polym. Sci. Polym. Chem. 2012, 50, 517.
[19] Nolan, E. M.; Lippard, S. J. Chem. Rev. 2008, 108, 3443.
[20] Yang, Y. M.; Zhao, Q.; Feng, W.; Li, F. Y. Chem. Rev. 2013, 113, 192.
[21] Zhao, Q.; Li, F. M.; Huang, C. H. Chem. Soc. Rev. 2010, 39, 3007.
[22] Quang, D. T.; Kim, J. S.; Yong, J. Chem. Soc. Rev. 2010, 110, 6280.
[23] Kim, H. N.; Ren, W. X.; Kim, J. S.; Yong, J. Chem. Soc. Rev. 2012, 41, 3210.
[24] Zhang, Y.; Li, W.; Wang, Q.; Zhang, R. X.; Xiong, Q. J.; Shen, X.; Guo, J.; Chen, X. M. Acta Chim. Sinica 2013, 71, 1496(in Chinese). (张勇, 李伟, 王强, 张若璇, 熊启杰, 沈祥, 郭靖, 陈雪梅, 化学学报, 2013, 71, 1496.)
[25] Zhang, C. Y.; Meng, Y. Z.; Kuang, J. Z.; Xu, L. Acta Chim. Sinica 2015, 73, 409(in Chinese). (张冲洋, 孟玉珠, 匡金志, 徐岚, 化学学报, 2015, 73, 409.)
[26] Wang, Q.; Yang, X. H.; Yang, X. H.; Liu, P.; Wang, K. M.; Huang, J.; Liu, J. B.; Song, C. X.; Wang, J. J. Spectrochim. Acta, Part A 2015, 136, 283.
[27] Liu, C. W.; Hsieh, Y. T.; Huang, C. C.; Lin, Z. H.; Chang, H. T. Chem. Commun. 2008, 19, 2242.
[28] Li, L.; Li, B. X.; Qi, Y. Y.; Jin, Y. Anal. Bioanal. Chem. 2009, 393, 2051.
[29] Guan, H. N.; Liu, X. F.; Wang, W.; Liang, J. Z. Spectrochim. Acta Part A 2014, 121, 527.
[30] Hung, Y. L.; Hsiung, T. M.; Chen, Y. Y.; Huang, Y. F.; Huang, C. C. J. Phys. Chem. C 2010, 114, 16329.
[31] Si, S.; Kotal, A.; Mandal, T. K. J. Phys. Chem. C 2007, 111, 1248.
[32] Ni, W. H.; Chen, H. J.; Su, J.; Sun, Z. H.; Wang, J. F.; Wu, H. K. J. Am. Chem. Soc. 2010, 132, 4806.
[33] Liu, D. B.; Qu, W. S.; Chen, W. W.; Zhang, W.; Wang, Z.; Jiang, X. Y. Anal. Chem. 2010, 82, 9606.
[34] Zhang, T. T.; Li, H.; Hou, S. W.; Dong, Y. Q.; Pang, G. S.; Zhang, Y. W. ACS Appl. Mater. Interfaces 2015, 7, 27131.
[35] Li, K.; Wang, K.; Qin, W. W.; Deng, S. H.; Li, D.; Shi, J. Y.; Huang, Q.; Fan, C. H. J. Am. Chem. Soc. 2015, 137, 4292.
/
| 〈 |
|
〉 |