

Advances on Nitrogen-centered Radical Chemistry:A Photocatalytic N-H Bond Activation Approach
Received date: 2017-08-23
Online published: 2017-09-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572140, 21732005) and the National Science and Technology Major Projects for "Major New Drugs Innovation and Development" (No. 2017ZX09101005-009-002).
Nitrogen-centered radicals are highly reactive intermediates, which provide new opportunities for designing new chemical reactions and preparing nitrogen-containing molecules. Direct generation of nitrogen-centered radicals via activation of N-H bonds under photocatalytic conditions has emerged as a green, efficient, and economical process, where significant progress has been made with methodology development in very recent years. In this paper, we highlight the important advances in this area that were reported since 2016.
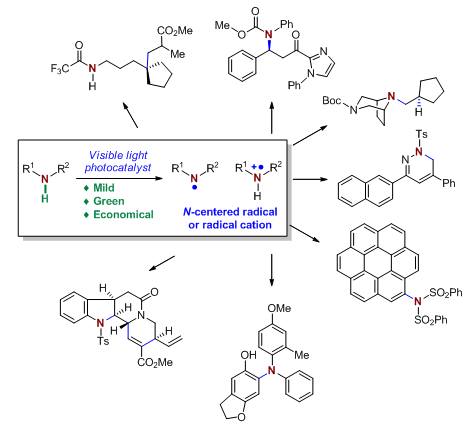
Song Hao , Liu Xiaoyu , Qin Yong . Advances on Nitrogen-centered Radical Chemistry:A Photocatalytic N-H Bond Activation Approach[J]. Acta Chimica Sinica, 2017 , 75(12) : 1137 -1149 . DOI: 10.6023/A17080384
[1] For selected reviews on the N-radical chemistry, see:
(a) Zard, S. Z. Chem. Soc. Rev. 2008, 37, 1603.
(b) Stella, L. In Radicals in Organic Synthesis, Vol. 2, Eds.: Renaud, P.; Sibi, M. P., Wiley, New York, 2001, p. 407.
(c) Quiclet-Sire, B.; Zard, S. Z. Beilstein J. Org. Chem. 2013, 9, 557.
[2] For selected examples, see:
(a) Lessard, J.; Cote, R.; Mackiewicz, P.; Furstoss, R.; Waegell, B. J. Org. Chem. 1978, 43, 3750.
(b) Boivin, J.; Fouquet, E.; Schiano, A.-M.; Zard, S. Z. Tetrahedron 1994, 50, 1769.
(c) Gennet, D.; Zard, S. Z.; Zhang, H. Chem. Commun. 2003, 1870.
(d) Lu, H.; Li, C. Tetrahedron Lett. 2005, 46, 5983.
(e) Guin, J.; Fröhlich, R.; Studer, A. Angew. Chem. Int. Ed. 2008, 47, 779.
[3] For selected examples, see:
(a) Sherman, E. S.; Chemler, S. R.; Tan, T. B.; Gerlits, O. Org. Lett. 2004, 6, 1573.
(b) Sherman, E. S.; Fuller, P. H.; Kasi, D.; Chemler, S. R. J. Org. Chem. 2007, 72, 3896.
(c) Zeng, W.; Chemler, S. R. J. Am. Chem. Soc. 2007, 129, 12948.
(d) Zhu, X.; Wang, Y.-F.; Ren, W.; Zhang, F.-L.; Chiba, S. Org. Lett. 2013, 15, 3214.
(e) Zhu, M.-K.; Chen, Y.-C.; Loh, T. P. Chem. Eur. J. 2013, 19, 5250.
(f) Duan, X.-Y.; Zhou, N.-N.; Fang, R.; Yang, X.-L.; Yu, W.; Han, B. Angew. Chem. Int. Ed. 2014, 53, 3158.
(g) Duan, X. Y.; Yang, X. L.; Jia, P. P.; Zhang, M.; Han, B. Org. Lett. 2015, 17, 6022.
[4] For selected reviews on the visible light photocatalysis, see:
(a) Xuan, J.; Xiao, W.-J. Angew. Chem. Int. Ed. 2012, 51, 6828.
(b) Shi, L.; Xia, W.-J. Chem. Soc. Rev. 2012, 41, 7687.
(c) Prier, C. K.; Rankic, D. A.; Macmillan, D. W. Chem. Rev. 2013, 113, 5322.
(d) Xi, Y.-M.; Yi, H.; Lei, A.-W. Org. Biomol. Chem. 2013, 11, 2387.
(e) Schultz, D. M.; Yoon, T. P. Science 2014, 343, 985.
(f) Xuan, J.; Zhang, Z.-G.; Xiao, W.-J. Angew. Chem. Int. Ed. 2015, 54, 15632.
(g) Karkas, M. D.; Porco Jr., J. A.; Stephenson, C. R. Chem. Rev. 2016, 116, 9683.
(h) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075.
(i) Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Acc. Chem. Res. 2016, 49, 1911.
(j) Liu, Y.; Song, R.; Li, J. Sci. China Chem. 2016, 59, 161.
(k) Zhang, J.; Chen, Y. Acta Chim. Sinica 2017, 75, 41(in Chinese). (张晶, 陈以昀, 化学学报, 2017, 75, 41.)
(l) Chen, J.-R.; Yan, D.-M.; Wei, Q.; Xiao, W.-J. ChemPhotoChem 2017, 1, 148.
[5] For recent reviews, see:
(a) Chen, J.-R.; Hu, X. Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044.
(b) Xiong, T.; Zhang, Q. Chem. Soc. Rev. 2016, 45, 3069.
(c) Gentry, E. C.; Knowles, R. R. Acc. Chem. Res. 2016, 49, 1546.
(d) Nguyen, L. Q.; Knowles, R. R. ACS Catal. 2016, 6, 2894.
(e) Kärkäs, M. D. ACS Catal. 2017, 7, 4999.
[6] For recent reviews, see:
(a) Gensch, T.; Hopkinson, M. N.; Glorius, F.; Wencel-Delord, J. Chem. Soc. Rev. 2016, 45, 2900.
(b) Zhu, R.-Y.; Farmer, M. E.; Chen, Y.-Q.; Yu, J.-Q. Angew. Chem. Int. Ed. 2016, 55, 10578.
(c) Della Ca', N.; Fontana, M.; Motti, E.; Catellani, M. Acc. Chem. Res. 2016, 49, 1389.
(d) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754.
[7] For selected examples, see:
(a) Qin, Q.; Yu, S. Org. Lett. 2015, 17, 1894.
(b) Hollister, K. A.; Conner, E. S.; Spell, M. L.; Deveaux, K.; Maneval, L.; Beal, M. W.; Ragains, J. R. Angew. Chem. Int. Ed. 2015, 54, 7837.
(c) Huang, F.-Q.; Dong, X.; Qi, L.-W.; Zhang, B. Tetrahedron Lett. 2016, 57, 1600.
(d) Zhang, J.; Li, Y.; Zhang, F.; Hu, C.; Chen, Y. Angew. Chem. Int. Ed. 2016, 55, 1872.
(e) Wang, C.; Harms, K.; Meggers, E. Angew. Chem. Int. Ed. 2016, 55, 13495.
(f) Shaaban, S.; Oh, J.; Maulide, N. Org. Lett. 2016, 18, 345.
(g) Parasram, M.; Chuentragool, P.; Sarkar, D.; Gevorgyan, V. J. Am. Chem. Soc. 2016, 138, 6340.
(h) Hu, X.-Q.; Chen, J.-R.; Xiao, W.-J. Angew. Chem. Int. Ed. 2017, 56, 1960.
[8] Choi, G. J.; Zhu, Q.; Miller, D. C.; Gu, C. J.; Knowles, R. R. Nature 2016, 539, 268.
[9] Chu, J. C. K.; Rovis, T. Nature 2016, 539, 272.
[10] Zhang, L.; Meggers, E. Acc. Chem. Res. 2017, 50, 320.
[11] Zhou, Z.; Li, Y.; Han, B.; Gong, L.; Meggers, E. Chem. Sci. 2017, 8, 5757.
[12] Yuan, W.; Zhou, Z.; Gong, L.; Meggers, E. Chem. Commun. 2017, 53, 8964.
[13] For a recent review, see: Huang, L.; Arndt, M.; Gooßen, K.; Heydt, H.; Gooßen, L. J. Chem. Rev. 2015, 115, 2596.
[14] Musacchio, A. J.; Lainhart, B. C.; Zhang, X.; Naguib, S. G.; Sherwood, T. C.; Knowles, R. R. Science 2017, 355, 727.
[15] For a recent review, see: Chen, Z.-M.; Zhang, X.-M.; Tu, Y.-Q. Chem. Soc. Rev. 2015, 44, 5220.
[16] Shu, W.; Genoux, A.; Li, Z.; Nevado, C. Angew. Chem. Int. Ed. 2017, 56, 10521.
[17]
(a) Hu, X.-Q.; Chen, J.-R.; Wei, Q.; Liu, F.-L.; Deng, Q.-H.; Beauchemin, A. M.; Xiao, W.-J. Angew. Chem. Int. Ed. 2014, 53, 12163.
(b) Hu, X.-Q.; Qi, X.; Chen, J.-R.; Zhao, Q.-Q.; Wei, Q.; Lan, Y.; Xiao, W.-J. Nat. Commun. 2016, 7, 11188.
(c) Zhao, Q.-Q.; Hu, X.-Q.; Yang, M.-N.; Chen, J.-R.; Xiao, W.-J. Chem. Commun. 2016, 52, 12749.
(d) Hu, X.-Q.; Chen, J.; Chen, J.-R.; Yan, D.-M.; Xiao, W.-J. Chem. Eur. J. 2016, 22, 14141.
(e) Zhao, Q.-Q.; Chen, J.; Yan, D.-M.; Chen, J.-R.; Xiao, W.-J. Org. Lett. 2017, 19, 3620.
(f) Yu, X.; Zhou, F.; Chen, J.; Xiao, W. Acta Chim. Sinica 2017, 75, 86(in Chinese). (余晓叶, 周帆, 陈加荣, 肖文精, 化学学报, 2017, 75, 86.)
[18] Tong, K.; Liu, X.; Zhang, Y.; Yu, S. Chem. Eur. J. 2016, 22, 15669.
[19] Ito, E.; Fukushima, T.; Kawakami, T.; Murakami, K.; Itami, K. Chem 2017, 2, 383.
[20]
(a) Zhao, Y.; Huang, B.; Yang, C.; Xia, W. Org. Lett. 2016, 18, 3326.
(b) Zhao, Y.; Huang, B.; Yang, C.; Li, B.; Gou, B.; Xia, W. ACS Catal. 2017, 7, 2446.
[21] Wang, X.; Xia, D.; Qin, W.; Zhou, R.; Zhou, X.; Zhou, Q.; Liu, W.; Dai, X.; Wang, H.; Wang, S.; Tan, L.; Zhang, D.; Song, H.; Liu, X.-Y.; Qin, Y. Chem 2017, 2, 803.
/
| 〈 |
|
〉 |